Alpha-synuclein suppression by targeted small interfering RNA in the primate substantia nigra - PubMed (original) (raw)
Alpha-synuclein suppression by targeted small interfering RNA in the primate substantia nigra
Alison L McCormack et al. PLoS One. 2010.
Abstract
The protein alpha-synuclein is involved in the pathogenesis of Parkinson's disease and other neurodegenerative disorders. Its toxic potential appears to be enhanced by increased protein expression, providing a compelling rationale for therapeutic strategies aimed at reducing neuronal alpha-synuclein burden. Here, feasibility and safety of alpha-synuclein suppression were evaluated by treating monkeys with small interfering RNA (siRNA) directed against alpha-synuclein. The siRNA molecule was chemically modified to prevent degradation by exo- and endonucleases and directly infused into the left substantia nigra. Results compared levels of alpha-synuclein mRNA and protein in the infused (left) vs. untreated (right) hemisphere and revealed a significant 40-50% suppression of alpha-synuclein expression. These findings could not be attributable to non-specific effects of siRNA infusion since treatment of a separate set of animals with luciferase-targeting siRNA produced no changes in alpha-synuclein. Infusion with alpha-synuclein siRNA, while lowering alpha-synuclein expression, had no overt adverse consequences. In particular, it did not cause tissue inflammation and did not change (i) the number and phenotype of nigral dopaminergic neurons, and (ii) the concentrations of striatal dopamine and its metabolites. The data represent the first evidence of successful anti-alpha-synuclein intervention in the primate substantia nigra and support further development of RNA interference-based therapeutics.
Conflict of interest statement
Competing Interests: One of the authors (D.B.) is an employee of Alnylam Pharmaceuticals, a for-profit company focused on the development of RNA interference-based therapeutics.
Figures
Figure 1. Treatment of squirrel monkeys with siRNA.
Animals were unilaterally implanted with a cannula connected to an Alzet minipump delivering siRNA into the left substantia nigra. (A) Sequence of the α-synuclein siRNA. “A,C,G,U” indicate ribonucleotides, “T” designates deoxythymidine, “c” and “u” specify 2′-O-Me-modified pyrimidines and “s” denotes a phosphorothioate linkage. (B) Midbrain sections were immunostained for tyrosine hydroxylase (brown) and counterstained with cresyl violet (purple). A representative section shows placement of the cannula approximately 1 mm dorsal to the substantia nigra (SN). The location of the cannula is indicated by the square box, and the asterisk denotes the exit of the third nerve. Scale bar = 800 µm.
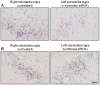
Figure 2. Reduction of α-synuclein mRNA in the substantia nigra infused with α-synuclein siRNA.
Squirrel monkeys received a unilateral nigral infusion of siRNA targeting α-synuclein (A) or luciferase (B). Midbrain sections at the level of the exit of the 3rd nerve were used for α-synuclein in situ hybridization using digoxigenin-labeled antisense riboprobes. Representative images compare α-synuclein mRNA in the right (untreated) vs. left (siRNA-infused) substantia nigra. Scale bar = 100 µm.
Figure 3. α-Synuclein siRNA decreases α-synuclein mRNA within pigmented nigral neurons.
siRNA against α-synuclein was infused into the left substantia nigra of squirrel monkeys. Right (A) and left (B) midbrain sections at the level of the exit of the 3rd nerve were used for α-synuclein in situ hybridization. Representative images show nigral dopaminergic neurons containing neuromelanin (brown granules). The hybridization signal (purple) was markedly reduced in the left (siRNA-infused) as compared to the right (untreated) hemisphere. Scale bar = 10 µm.
Figure 4. Measurement of nigral α-synuclein mRNA by qPCR.
Squirrel monkeys received unilateral nigral infusion of siRNA targeting α-synuclein (A) or luciferase (B). Nigral tissue was dissected from midbrain sections rostral and caudal to the exit of the 3rd nerve. Values are the ratio of α-synuclein mRNA levels measured by qPCR in the left (siRNA-infused) and right (untreated) substantia nigra (L∶R ratio). Bars represent mean values.
Figure 5. Effect of α-synuclein siRNA on α-synuclein protein in the monkey substantia nigra.
α-Synuclein or luciferase siRNA was unilaterally infused into the left substantia nigra. Midbrain sections were immunostained with an antibody against α-synuclein. Representative images from an animal receiving α-synuclein siRNA show more robust α-synuclein immunoreactivity within the neuropil of the right (untreated, A) vs. left (siRNA-infused, B) substantia nigra. Scale bar = 5 µm. (C) Optical density measurements of nigral α-synuclein immunoreactivity. Data are expressed as percent of the control value in the right (untreated) substantia nigra and represent mean ± SEM. A significant decrease is caused by α-synuclein but not luciferase siRNA in the left (siRNA-infused) hemisphere. *p<0.03.
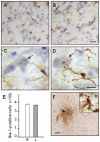
Figure 6. Lack of microglial activation following siRNA infusion.
α-Synuclein siRNA was unilaterally infused through a cannula positioned approximately 1 mm dorsal to the substantia nigra. Representative midbrain sections were immunostained for microglial cells using an antibody against ionizing calcium-binding adaptor molecule 1 (Iba-1, brown) and counterstained with cresyl violet (purple). Images are from the right (untreated, A and C) and left (siRNA-infused, B and D) substantia nigra. At higher magnification (C and D), Iba-1-positive cells with morphological features of resting microglia are shown close to dopaminergic neurons containing neuromelanin (black granules). The arrows indicate one of these neurons in each panel. Scale bars = 20 µm (A and B) and 10 µm (C and D). (E) The number of Iba-1-immunoreactive cells was counted in the right (R) and left (L) substantia nigra. Data are shown as mean ± SEM. (F) A representative section from the left midbrain shows Iba-1 immunoreactivity close to the tip of the infusion cannula (arrow) but not within the nearby parenchyma. This robust immunoreactivity was observed within cells with morphological characteristics of activated microglia (inset). Scale bars = 250 µm (panel F) and 10 µm (inset).
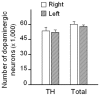
Figure 7. The number of nigral dopaminergic neurons is not affected by siRNA-induced α-synuclein suppression.
Squirrel monkeys received a unilateral nigral infusion of siRNA targeting α-synuclein. Both the number of TH-immunoreactive cells and the total number of dopaminergic neurons were counted stereologically in the substantia nigra. Values (mean ± SEM) were not different between the right (untreated) and left (siRNA-infused) hemisphere.
Similar articles
- Selective α-Synuclein Knockdown in Monoamine Neurons by Intranasal Oligonucleotide Delivery: Potential Therapy for Parkinson's Disease.
Alarcón-Arís D, Recasens A, Galofré M, Carballo-Carbajal I, Zacchi N, Ruiz-Bronchal E, Pavia-Collado R, Chica R, Ferrés-Coy A, Santos M, Revilla R, Montefeltro A, Fariñas I, Artigas F, Vila M, Bortolozzi A. Alarcón-Arís D, et al. Mol Ther. 2018 Feb 7;26(2):550-567. doi: 10.1016/j.ymthe.2017.11.015. Epub 2017 Nov 29. Mol Ther. 2018. PMID: 29273501 Free PMC article. - Alpha-synuclein expression in the substantia nigra of MPTP-lesioned non-human primates.
Purisai MG, McCormack AL, Langston WJ, Johnston LC, Di Monte DA. Purisai MG, et al. Neurobiol Dis. 2005 Dec;20(3):898-906. doi: 10.1016/j.nbd.2005.05.028. Epub 2005 Jul 11. Neurobiol Dis. 2005. PMID: 16006134 - Long-term RNAi knockdown of α-synuclein in the adult rat substantia nigra without neurodegeneration.
Zharikov A, Bai Q, De Miranda BR, Van Laar A, Greenamyre JT, Burton EA. Zharikov A, et al. Neurobiol Dis. 2019 May;125:146-153. doi: 10.1016/j.nbd.2019.01.004. Epub 2019 Jan 15. Neurobiol Dis. 2019. PMID: 30658149 Free PMC article. - Dopaminergic neuron loss and up-regulation of chaperone protein mRNA induced by targeted over-expression of alpha-synuclein in mouse substantia nigra.
St Martin JL, Klucken J, Outeiro TF, Nguyen P, Keller-McGandy C, Cantuti-Castelvetri I, Grammatopoulos TN, Standaert DG, Hyman BT, McLean PJ. St Martin JL, et al. J Neurochem. 2007 Mar;100(6):1449-57. doi: 10.1111/j.1471-4159.2006.04310.x. Epub 2007 Jan 4. J Neurochem. 2007. PMID: 17241127 - shRNA targeting α-synuclein prevents neurodegeneration in a Parkinson's disease model.
Zharikov AD, Cannon JR, Tapias V, Bai Q, Horowitz MP, Shah V, El Ayadi A, Hastings TG, Greenamyre JT, Burton EA. Zharikov AD, et al. J Clin Invest. 2015 Jul 1;125(7):2721-35. doi: 10.1172/JCI64502. Epub 2015 Jun 15. J Clin Invest. 2015. PMID: 26075822 Free PMC article.
Cited by
- Nuclease-dead S. aureus Cas9 downregulates alpha-synuclein and reduces mtDNA damage and oxidative stress levels in patient-derived stem cell model of Parkinson's disease.
Sastre D, Zafar F, Torres CAM, Piper D, Kirik D, Sanders LH, Qi S, Schüle B. Sastre D, et al. bioRxiv [Preprint]. 2023 Jan 24:2023.01.24.525105. doi: 10.1101/2023.01.24.525105. bioRxiv. 2023. PMID: 36747875 Free PMC article. Updated. Preprint. - Noninvasive delivery of an α-synuclein gene silencing vector with magnetic resonance-guided focused ultrasound.
Xhima K, Nabbouh F, Hynynen K, Aubert I, Tandon A. Xhima K, et al. Mov Disord. 2018 Oct;33(10):1567-1579. doi: 10.1002/mds.101. Epub 2018 Sep 28. Mov Disord. 2018. PMID: 30264465 Free PMC article. - Does α-synuclein have a dual and opposing effect in preclinical vs. clinical Parkinson's disease?
Markopoulou K, Biernacka JM, Armasu SM, Anderson KJ, Ahlskog JE, Chase BA, Chung SJ, Cunningham JM, Farrer M, Frigerio R, Maraganore DM. Markopoulou K, et al. Parkinsonism Relat Disord. 2014 Jun;20(6):584-9; discussion 584. doi: 10.1016/j.parkreldis.2014.02.021. Epub 2014 Mar 5. Parkinsonism Relat Disord. 2014. PMID: 24656894 Free PMC article. - Nucleic Acid-Based Therapeutics for Parkinson's Disease.
Nakamori M, Junn E, Mochizuki H, Mouradian MM. Nakamori M, et al. Neurotherapeutics. 2019 Apr;16(2):287-298. doi: 10.1007/s13311-019-00714-7. Neurotherapeutics. 2019. PMID: 30756362 Free PMC article. Review. - Trends on Novel Targets and Nanotechnology-Based Drug Delivery System in the Treatment of Parkinson's disease: Recent Advancement in Drug Development.
Majumdar M, Badwaik H. Majumdar M, et al. Curr Drug Targets. 2024;25(15):987-1011. doi: 10.2174/0113894501312703240826070530. Curr Drug Targets. 2024. PMID: 39313872 Review.
References
- Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, et al. Mutation in the α-synuclein gene identified in families with Parkinson's disease. Science. 1997;276:2045–2047. - PubMed
- Kruger R, Kuhn W, Muller T, Woitalla D, Graeber M, et al. Ala30Pro mutation in the gene encoding α-synuclein in Parkinson's disease. Nat Genet. 1998;18:106–108. - PubMed
- Singleton AB, Farrer M, Johnson J, Singleton A, Hague S, et al. α-Synuclein locus triplication causes Parkinson's disease. Science. 2003;302:841. - PubMed
- Zarranz JJ, Alegre J, Gomez-Esteban JC, Lezcano E, Ros R, et al. The new mutation, E46K, of α-synuclein causes Parkinson and Lewy body dementia. Ann Neurol. 2004;55:164–173. - PubMed
- Farrer M, Kachergus J, Forno L, Lincoln S, Wang DS, et al. Comparison of kindreds with parkinsonism and α-synuclein genomic multiplications. Ann Neurol. 2004;55:174–179. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources