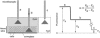Microfiberoptic measurement of extracellular space volume in brain and tumor slices based on fluorescent dye partitioning - PubMed (original) (raw)
Microfiberoptic measurement of extracellular space volume in brain and tumor slices based on fluorescent dye partitioning
Hua Zhang et al. Biophys J. 2010.
Abstract
The fractional volume occupied by extracellular space in tissues, termed alpha, is an important parameter of tissue architecture that affects cellular functions and drug delivery. We report a technically simple fluorescent dye partitioning method to measure alpha in tissue slices based on microfiberoptic detection of dye fluorescence in tissue versus overlying solution. Microfiberoptic tip geometry and dyes were selected for alpha determination from fluorescence intensity ratios, without the need to correct for illumination profile, light scattering/absorption, or dye binding. The method was validated experimentally using cell-embedded gels of specified alpha-values and optical properties. In mouse brain slices, alpha was strongly location-dependent, ranging from 0.16 in thalamus to 0.22 in brainstem, and was sensitive to cell volume changes. Aquaporin-4 water channel gene deletion caused significant extracellular space expansion, with alpha = 0.181 +/- 0.002 in cortex in wild-type mice and 0.211 +/- 0.003 in Aquaporin-4 knockout mice. In slices of LLC1 cell tumors grown in mice to approximately 5 mm diameter, alpha decreased remarkably from approximately 0.45 in superficial tumor to <0.25 in deeper (>100 mum) tumor. Fluorescent dye partitioning with microfiberoptic detection permits rapid, accurate, and anisotropy-insensitive determination of alpha-values in tissue slices.
2010 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures
Figure 1
Principle of α determination by DPMD. Left: Microfiberoptic detection of the fluorescence of a noninteracting, aqueous-phase dye in the tissue slice and overlying solution. The illumination/detection volume is approximately conical, with most of the light collected from near the fiberoptic tip. Right: Schematic of DPMD data showing α determination from the ratio of the (background-subtracted) dye fluorescence in the slice to that in the overlying solution.
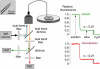
Figure 2
Instrumentation and procedure for α determination by DPMD. Left: A multimode etched microfiberoptic with micron-sized tip is inserted into the overlying solution and tissue slice. Inset shows the microfiberoptic tip geometry. Fluorescence from two probes of different colors is detected simultaneously using two photomultipliers (PMT). Right: Relative fluorescence of calcein (green) and rhod-dextran (red; normalized to unity in the overlying solution and zero in water) during insertion of the microfiberoptic into the overlying solution and into a brain slice (in hippocampus). Fluorescence ratios gave α = 0.21 for both colors.
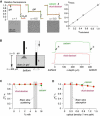
Figure 3
Experimental validation using cell-embedded gels and solutions simulating tissue optical properties. (A) Agarose gels containing SP2/0 cells giving specified α were bathed in PBS containing calcein and rhod-dextran. Top: Dye fluorescence shown during microfiberoptic insertion into the overlying solutions and gels. Bottom: Brightfield photographs show different cell densities, giving α = 1 (gel not containing cells), 0.5, and 0.3. Bar: 50 _μ_m. Right: Experimentally measured (from fluorescence ratios) versus calculated (from cell density and size) α (SE, n = 4, except n = 2 for α = 0.15). (B) Effects of illumination/detection geometry. Left: A coverglass supports a 400-_μ_m-thick agarose gel equilibrated with calcein and rhod-dextran in PBS. Right: Fluorescence shown during insertion of the microfiberoptic through the gel down to the bottom surface. The example is representative of six separate experiments. (C) Effect of light scattering. PBS/dye solutions were supplemented with nonfat milk (up to 16%) to produce light scattering. Calcein and rhod-dextran fluorescence changed by <1% for <12% milk (equivalent scattering from brain slices 7–10% milk, SE, n = 6 for each point). (D) Effect of light absorbance. PBS/dye solutions were supplemented with India ink (0–0.5%) to produce light absorbance (optical density at 1 mm pathlength from 0 to 1.5 at 595 nm). Calcein and rhod-dextran fluorescence changed by <1% for 0.2% India ink solution (equivalent optical density from brain slices: 0.3; SE, n = 6 for each point).
Figure 4
ECS volume measurements in brain slices from mice. (A) Equilibration time course of fluorescence after dye addition to the bathing solution. Microfiberoptic tip positioned at a depth of 200 _μ_m in 400-_μ_m-thick brain slices (SE, n = 5). (B) Washout time course of fluorescence (SE, n = 4). (C) Fluorescence of calcein and rhod-dextran during repeated microfiberoptic insertion into a brain slice (in cortex at 400 _μ_m from brain surface). (D) Kinetics of changes in α in response to brain cell volume-altering maneuvers, including addition of 50 mM mannitol (top), 100 _μ_M NMDA (bottom), 50 mM high K+ (bottom), and 100 _μ_M ouabain (bottom). Control slices with stable α shown for comparison (labeled aCSF). Examples are representative of four or more separate experiments.
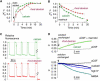
Figure 5
Regional variation of α in brain, and effects of AQP4 gene deletion. (A) Top: Photograph of brain slices with indicated measurement regions (bar: 1 mm). Bottom: Regional differences in α. Summary of α (SE, 6–10 slices per location, ∗p < 0.05, ∗∗p < 0.001) in indicated regions. (B) Effects of AQP4 gene deletion. Top: DPMD data comparing brain slices from WT and AQP4 null mice. Bottom: Summary of α in individual mice (SE, four slices per mouse, six measurements in cortical gray matter per slice), and averaged α for all slices (SE, four mice, ∗p < 0.001).
Figure 6
ECS volume measurement in tumor slices. Slices cut from a 5-mm-diameter subcutaneous tumor in a mouse at 12 days after injection of LLC1 cells. (A) Histology showing hematoxylin and eosin staining of a tumor slice in a direction transverse to the skin surface. Squares indicate sites of microfiberoptic insertion. Bar: 500 _μ_m. Panels a–f show high-magnification views of microfiberoptic insertion areas. Bar: 50 μ_m. (B) Summary of α_-values at different depths from the tumor surface (SE, 8–10 slices per location, ∗_p < 0.05, ∗∗_p < 0.001 compared with α at 50 _μ_m). Representative of studies in tumors from four mice.
Similar articles
- Microfiberoptic fluorescence photobleaching reveals size-dependent macromolecule diffusion in extracellular space deep in brain.
Zador Z, Magzoub M, Jin S, Manley GT, Papadopoulos MC, Verkman AS. Zador Z, et al. FASEB J. 2008 Mar;22(3):870-9. doi: 10.1096/fj.07-9468com. Epub 2007 Oct 26. FASEB J. 2008. PMID: 17965267 - Extracellular space volume measured by two-color pulsed dye infusion with microfiberoptic fluorescence photodetection.
Magzoub M, Zhang H, Dix JA, Verkman AS. Magzoub M, et al. Biophys J. 2009 Mar 18;96(6):2382-90. doi: 10.1016/j.bpj.2008.12.3916. Biophys J. 2009. PMID: 19289063 Free PMC article. - Slowed diffusion in tumors revealed by microfiberoptic epifluorescence photobleaching.
Thiagarajah JR, Kim JK, Magzoub M, Verkman AS. Thiagarajah JR, et al. Nat Methods. 2006 Apr;3(4):275-80. doi: 10.1038/nmeth863. Nat Methods. 2006. PMID: 16554832 - Enlarged extracellular space of aquaporin-4-deficient mice does not enhance diffusion of Alexa Fluor 488 or dextran polymers.
Xiao F, Hrabetová S. Xiao F, et al. Neuroscience. 2009 Jun 16;161(1):39-45. doi: 10.1016/j.neuroscience.2009.03.017. Epub 2009 Mar 19. Neuroscience. 2009. PMID: 19303428 Free PMC article. - Fluorescence detection of redox-sensitive metals in neuronal culture: focus on iron and zinc.
Reynolds IJ. Reynolds IJ. Ann N Y Acad Sci. 2004 Mar;1012:27-36. doi: 10.1196/annals.1306.003. Ann N Y Acad Sci. 2004. PMID: 15105253 Review.
Cited by
- Intercellular Communication in the Central Nervous System as Deduced by Chemical Neuroanatomy and Quantitative Analysis of Images: Impact on Neuropharmacology.
Guidolin D, Tortorella C, Marcoli M, Maura G, Agnati LF. Guidolin D, et al. Int J Mol Sci. 2022 May 22;23(10):5805. doi: 10.3390/ijms23105805. Int J Mol Sci. 2022. PMID: 35628615 Free PMC article. Review. - Brain clearance is reduced during sleep and anesthesia.
Miao A, Luo T, Hsieh B, Edge CJ, Gridley M, Wong RTC, Constandinou TG, Wisden W, Franks NP. Miao A, et al. Nat Neurosci. 2024 Jun;27(6):1046-1050. doi: 10.1038/s41593-024-01638-y. Epub 2024 May 13. Nat Neurosci. 2024. PMID: 38741022 Free PMC article. - Infrared spectroscopic studies of cells and tissues: triple helix proteins as a potential biomarker for tumors.
Stelling AL, Toher D, Uckermann O, Tavkin J, Leipnitz E, Schweizer J, Cramm H, Steiner G, Geiger KD, Kirsch M. Stelling AL, et al. PLoS One. 2013;8(3):e58332. doi: 10.1371/journal.pone.0058332. Epub 2013 Mar 20. PLoS One. 2013. PMID: 23526977 Free PMC article. - Diffusion in the extracellular space in brain and tumors.
Verkman AS. Verkman AS. Phys Biol. 2013 Aug;10(4):045003. doi: 10.1088/1478-3975/10/4/045003. Epub 2013 Aug 2. Phys Biol. 2013. PMID: 23913007 Free PMC article. - Greatly improved survival and neuroprotection in aquaporin-4-knockout mice following global cerebral ischemia.
Katada R, Akdemir G, Asavapanumas N, Ratelade J, Zhang H, Verkman AS. Katada R, et al. FASEB J. 2014 Feb;28(2):705-14. doi: 10.1096/fj.13-231274. Epub 2013 Nov 1. FASEB J. 2014. PMID: 24186965 Free PMC article.
References
- Jain R.K. Delivery of molecular and cellular medicine to solid tumors. Adv. Drug Deliv. Rev. 1997;26:71–90. - PubMed
- Law R.O. Techniques and applications of extracellular space determination in mammalian tissues. Experientia. 1982;38:411–421. - PubMed
- Syková E. Diffusion properties of the brain in health and disease. Neurochem. Int. 2004;45:453–466. - PubMed
- Nicholson C., Syková E. Extracellular space structure revealed by diffusion analysis. Trends Neurosci. 1998;21:207–215. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- HL73856/HL/NHLBI NIH HHS/United States
- R01 EY013574/EY/NEI NIH HHS/United States
- DK86125/DK/NIDDK NIH HHS/United States
- R01 EB000415/EB/NIBIB NIH HHS/United States
- R01 DK035124/DK/NIDDK NIH HHS/United States
- DK72517/DK/NIDDK NIH HHS/United States
- EY13574/EY/NEI NIH HHS/United States
- DK35124/DK/NIDDK NIH HHS/United States
- R01 HL073856/HL/NHLBI NIH HHS/United States
- P30 DK072517/DK/NIDDK NIH HHS/United States
- RC1 DK086125/DK/NIDDK NIH HHS/United States
- EB00415/EB/NIBIB NIH HHS/United States
- R37 DK035124/DK/NIDDK NIH HHS/United States
- R37 EB000415/EB/NIBIB NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical