Toll-like receptor 3 inhibits memory retention and constrains adult hippocampal neurogenesis - PubMed (original) (raw)
Toll-like receptor 3 inhibits memory retention and constrains adult hippocampal neurogenesis
Eitan Okun et al. Proc Natl Acad Sci U S A. 2010.
Abstract
Toll-like receptors (TLRs) are innate immune receptors that have recently emerged as regulators of neuronal survival and developmental neuroplasticity. Adult TLR3-deficient mice exhibited enhanced hippocampus-dependent working memory in the Morris water maze, novel object recognition, and contextual fear-conditioning tasks. In contrast, TLR3-deficient mice demonstrated impaired amygdala-related behavior and anxiety in the cued fear-conditioning, open field, and elevated plus maze tasks. Further, TLR3-deficient mice exhibited increased hippocampal CA1 and dentate gyrus volumes, increased hippocampal neurogenesis, and elevated levels of the AMPA receptor subunit GluR1 in the CA1 region of the hippocampus. In addition, levels of activated forms of the kinase ERK and the transcription factor CREB were elevated in the hippocampus of TLR3-deficient mice, suggesting that constitutive TLR3 signaling negatively regulates pathways known to play important roles in hippocampal plasticity. Direct activation of TLR3 by intracerebroventricular infusion of a TLR3 ligand impaired working memory, but not reference memory. Our findings reveal previously undescribed roles for TLR3 as a suppressor of hippocampal cellular plasticity and memory retention.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
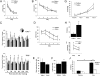
Hippocampus-dependent working memory is enhanced and amygdala-dependent memory is impaired in mice lacking TLR3. (A) TLR3+/+ (n = 16) and TLR3−/− (n = 16) mice show similar latencies to reach a visible platform in a nonspatial variant of the MWM. (B) TLR3+/+ and TLR3−/− mice exhibit similar learning rates, as measured by their escape latencies. (C) Whereas TLR3+/+ mice retain memory of the platform up to 72 h; TLR3−/− mice retain memory of the platform location up to 120 h posttraining. (D) TLR3−/− mice exhibit enhanced working memory acquisition compared with TLR3+/+ mice. As early as day 2 of the test, TLR3−/− show shorter latencies to find the platform in its new location. (E) No significant difference in total exploration during object familiarization between TLR3+/+ and TLR3−/− mice. (F) TLR3−/− mice exhibit significantly higher preference for a novel object than TLR3+/+ mice in the novel object recognition test. (G) TLR3+/+ and TLR3−/− mice show similar association curves in the fear-conditioning paradigm. (H) TLR3−/− mice exhibit significantly greater hippocampus-dependent contextual fear than TLR3+/+ mice, as measured by time freezing. (I) TLR3−/− mice exhibit slower contextual memory extinction than TLR3+/+ mice. (J) TLR3−/− mice exhibit impaired cued fear and freeze less than TLR3+/+ mice in the presence of tone.
Fig. 2.
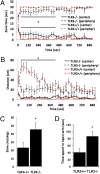
TLR3−/− mice exhibit reduced anxiety-like behaviors. (A) TLR3−/− (n = 16) mice spend more time in the center of the arena compared with TLR3+/+ (n = 16) mice. (B) TLR3−/− mice cover less distance in the periphery of the arena compared with TLR3+/+ mice. (C) TLR3−/− mice cross more between the center and peripheral zones in an open field. (D) TLR3−/− mice spend more time than TLR3+/+ mice in the open arm in an elevated plus maze.
Fig. 3.
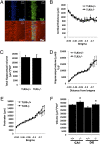
Loss of TLR3 alters hippocampal structure. (A) TLR3 is expressed in the CA1 in adult TLR3+/+ mice. (B_–_E) Cortical thickness (B), hippocampal volume (C and D), and hippocampal perimeter (E) were similar in TLR3+/+ (n = 6) and TLR3−/− (n = 6) mice. (F) TLR3−/− mice exhibit increased DG and CA1 volumes.
Comment in
- Neuroimmunology: Working memory takes its toll.
Hutchinson E. Hutchinson E. Nat Rev Neurosci. 2010 Oct;11(10):664. doi: 10.1038/nrn2917. Nat Rev Neurosci. 2010. PMID: 21080530 No abstract available.
Similar articles
- Evidence for a developmental role for TLR4 in learning and memory.
Okun E, Barak B, Saada-Madar R, Rothman SM, Griffioen KJ, Roberts N, Castro K, Mughal MR, Pita MA, Stranahan AM, Arumugam TV, Mattson MP. Okun E, et al. PLoS One. 2012;7(10):e47522. doi: 10.1371/journal.pone.0047522. Epub 2012 Oct 11. PLoS One. 2012. PMID: 23071817 Free PMC article. - Acutely increasing δGABA(A) receptor activity impairs memory and inhibits synaptic plasticity in the hippocampus.
Whissell PD, Eng D, Lecker I, Martin LJ, Wang DS, Orser BA. Whissell PD, et al. Front Neural Circuits. 2013 Sep 17;7:146. doi: 10.3389/fncir.2013.00146. eCollection 2013. Front Neural Circuits. 2013. PMID: 24062648 Free PMC article. - Increasing adult hippocampal neurogenesis is sufficient to improve pattern separation.
Sahay A, Scobie KN, Hill AS, O'Carroll CM, Kheirbek MA, Burghardt NS, Fenton AA, Dranovsky A, Hen R. Sahay A, et al. Nature. 2011 Apr 28;472(7344):466-70. doi: 10.1038/nature09817. Epub 2011 Apr 3. Nature. 2011. PMID: 21460835 Free PMC article. - Functions of adult-born neurons in hippocampal memory interference and indexing.
Miller SM, Sahay A. Miller SM, et al. Nat Neurosci. 2019 Oct;22(10):1565-1575. doi: 10.1038/s41593-019-0484-2. Epub 2019 Sep 2. Nat Neurosci. 2019. PMID: 31477897 Free PMC article. Review. - The role of the GluR-A (GluR1) AMPA receptor subunit in learning and memory.
Sanderson DJ, Good MA, Seeburg PH, Sprengel R, Rawlins JN, Bannerman DM. Sanderson DJ, et al. Prog Brain Res. 2008;169:159-78. doi: 10.1016/S0079-6123(07)00009-X. Prog Brain Res. 2008. PMID: 18394473 Review.
Cited by
- Evidence for a developmental role for TLR4 in learning and memory.
Okun E, Barak B, Saada-Madar R, Rothman SM, Griffioen KJ, Roberts N, Castro K, Mughal MR, Pita MA, Stranahan AM, Arumugam TV, Mattson MP. Okun E, et al. PLoS One. 2012;7(10):e47522. doi: 10.1371/journal.pone.0047522. Epub 2012 Oct 11. PLoS One. 2012. PMID: 23071817 Free PMC article. - Nanoligomers targeting NF-κB and NLRP3 reduce neuroinflammation and improve cognitive function with aging and tauopathy.
Wahl D, Risen SJ, Osburn SC, Emge T, Sharma S, Gilberto VS, Chatterjee A, Nagpal P, Moreno JA, LaRocca TJ. Wahl D, et al. J Neuroinflammation. 2024 Jul 27;21(1):182. doi: 10.1186/s12974-024-03182-9. J Neuroinflammation. 2024. PMID: 39068433 Free PMC article. - The regulatory role of Toll-like receptors after ischemic stroke: neurosteroids as TLR modulators with the focus on TLR2/4.
Tajalli-Nezhad S, Karimian M, Beyer C, Atlasi MA, Azami Tameh A. Tajalli-Nezhad S, et al. Cell Mol Life Sci. 2019 Feb;76(3):523-537. doi: 10.1007/s00018-018-2953-2. Epub 2018 Oct 30. Cell Mol Life Sci. 2019. PMID: 30377701 Free PMC article. Review. - The reverse transcriptase inhibitor 3TC protects against age-related cognitive dysfunction.
Wahl D, Smith ME, McEntee CM, Cavalier AN, Osburn SC, Burke SD, Grant RA, Nerguizian D, Lark DS, Link CD, LaRocca TJ. Wahl D, et al. Aging Cell. 2023 May;22(5):e13798. doi: 10.1111/acel.13798. Epub 2023 Mar 22. Aging Cell. 2023. PMID: 36949552 Free PMC article. - Toll-like receptors in the depressed and suicide brain.
Pandey GN, Rizavi HS, Ren X, Bhaumik R, Dwivedi Y. Pandey GN, et al. J Psychiatr Res. 2014 Jun;53:62-8. doi: 10.1016/j.jpsychires.2014.01.021. Epub 2014 Feb 11. J Psychiatr Res. 2014. PMID: 24565447 Free PMC article.
References
- Kawai T, Akira S. Signaling to NF-kappaB by Toll-like receptors. Trends Mol Med. 2007;13:460–469. - PubMed
- Zhou H, Lapointe BM, Clark SR, Zbytnuik L, Kubes P. A requirement for microglial TLR4 in leukocyte recruitment into brain in response to lipopolysaccharide. J Immunol. 2006;177:8103–8110. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous