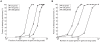Common inherited variation in mitochondrial genes is not enriched for associations with type 2 diabetes or related glycemic traits - PubMed (original) (raw)
Meta-Analysis
Common inherited variation in mitochondrial genes is not enriched for associations with type 2 diabetes or related glycemic traits
Ayellet V Segrè et al. PLoS Genet. 2010.
Abstract
Mitochondrial dysfunction has been observed in skeletal muscle of people with diabetes and insulin-resistant individuals. Furthermore, inherited mutations in mitochondrial DNA can cause a rare form of diabetes. However, it is unclear whether mitochondrial dysfunction is a primary cause of the common form of diabetes. To date, common genetic variants robustly associated with type 2 diabetes (T2D) are not known to affect mitochondrial function. One possibility is that multiple mitochondrial genes contain modest genetic effects that collectively influence T2D risk. To test this hypothesis we developed a method named Meta-Analysis Gene-set Enrichment of variaNT Associations (MAGENTA; http://www.broadinstitute.org/mpg/magenta). MAGENTA, in analogy to Gene Set Enrichment Analysis, tests whether sets of functionally related genes are enriched for associations with a polygenic disease or trait. MAGENTA was specifically designed to exploit the statistical power of large genome-wide association (GWA) study meta-analyses whose individual genotypes are not available. This is achieved by combining variant association p-values into gene scores and then correcting for confounders, such as gene size, variant number, and linkage disequilibrium properties. Using simulations, we determined the range of parameters for which MAGENTA can detect associations likely missed by single-marker analysis. We verified MAGENTA's performance on empirical data by identifying known relevant pathways in lipid and lipoprotein GWA meta-analyses. We then tested our mitochondrial hypothesis by applying MAGENTA to three gene sets: nuclear regulators of mitochondrial genes, oxidative phosphorylation genes, and approximately 1,000 nuclear-encoded mitochondrial genes. The analysis was performed using the most recent T2D GWA meta-analysis of 47,117 people and meta-analyses of seven diabetes-related glycemic traits (up to 46,186 non-diabetic individuals). This well-powered analysis found no significant enrichment of associations to T2D or any of the glycemic traits in any of the gene sets tested. These results suggest that common variants affecting nuclear-encoded mitochondrial genes have at most a small genetic contribution to T2D susceptibility.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Figure 1. Description of Meta-Analysis Gene-set Enrichment of variaNT Associations (MAGENTA) method.
(A) Step 1: Map genetic variants and their association scores onto genes. MAGENTA uses as input the association z-scores or _p_-values of DNA sequence variants across the entire genome. In this work, we used association _p_-values of single-nucleotide polymorphisms, SNPs (circles) from a genome-wide association study or meta-analysis, denoted as  for SNP i. Gene boundaries (vertical dashed lines) are defined here as predetermined physical distances added upstream and downstream to the most extreme transcript start and end sites of the gene (red arrow), respectively. Linkage-based distances can also be used. Each gene is assigned a set of SNPs that fall in its gene region boundaries. Two genes are shown for simplicity. (B) Step 2: Score genes based on their local SNP
for SNP i. Gene boundaries (vertical dashed lines) are defined here as predetermined physical distances added upstream and downstream to the most extreme transcript start and end sites of the gene (red arrow), respectively. Linkage-based distances can also be used. Each gene is assigned a set of SNPs that fall in its gene region boundaries. Two genes are shown for simplicity. (B) Step 2: Score genes based on their local SNP  . Here the most significant
. Here the most significant  of all SNPs i that lie within the extended gene boundaries is assigned to each gene g in the genome (
of all SNPs i that lie within the extended gene boundaries is assigned to each gene g in the genome ( ). (C) Step 3: Correct for confounding effects on the gene score,
). (C) Step 3: Correct for confounding effects on the gene score,  in the absence of genotype data. In this study we used step-wise multivariate linear regression analysis to regress out of
in the absence of genotype data. In this study we used step-wise multivariate linear regression analysis to regress out of  the confounding effects of several physical and genetic properties of genes (listed in Table 1);
the confounding effects of several physical and genetic properties of genes (listed in Table 1);  refers to the corrected gene _p_-value for gene g. In cases where two genes are assigned the same best SNP _p_-value,
refers to the corrected gene _p_-value for gene g. In cases where two genes are assigned the same best SNP _p_-value,  tends to be more significant for small genes than for large genes. (D) Step 4: Calculate a gene set enrichment _p_-value for each biological pathway or gene set of interest. We used a non-parametric statistical test to test whether
tends to be more significant for small genes than for large genes. (D) Step 4: Calculate a gene set enrichment _p_-value for each biological pathway or gene set of interest. We used a non-parametric statistical test to test whether  for all genes in gene set gs are enriched for highly ranked gene scores more than would be expected by chance, compared to randomly sampled gene sets of identical size from the genome.
for all genes in gene set gs are enriched for highly ranked gene scores more than would be expected by chance, compared to randomly sampled gene sets of identical size from the genome.  refers to the nominal gene set enrichment _p_-value for gene set gs.
refers to the nominal gene set enrichment _p_-value for gene set gs.
Figure 2. Regression analysis corrects for majority of confounding effects on gene association scores in a genotype-independent manner.
The performance of a step-wise regression analysis approach in correcting for confounders on  was evaluated against permutation analysis correction, since the latter corrects for all confounders without requiring a priori knowledge of them. T2D gene association _p_-values were plotted for all genes g in the genome (A) before gene score adjustment (
was evaluated against permutation analysis correction, since the latter corrects for all confounders without requiring a priori knowledge of them. T2D gene association _p_-values were plotted for all genes g in the genome (A) before gene score adjustment ( ) and (B) after correction for confounders using regression analysis (
) and (B) after correction for confounders using regression analysis ( ), as a function of corrected gene _p_-values using phenotype permutation analysis (
), as a function of corrected gene _p_-values using phenotype permutation analysis ( ). The Diabetes Genetics Initiative (DGI) GWA study was used for the analysis, since we had access to all individuals' genotypes.
). The Diabetes Genetics Initiative (DGI) GWA study was used for the analysis, since we had access to all individuals' genotypes.  is the association _p_-value of the best regional SNP for gene g before correction (y-axis in A). To compute
is the association _p_-value of the best regional SNP for gene g before correction (y-axis in A). To compute  (y-axis in B), step-wise multivariate linear regression analysis was applied to
(y-axis in B), step-wise multivariate linear regression analysis was applied to  against the first four confounders listed in Table 1 (this approach does not require genotype data). The Pearson's correlation coefficient (calculated between _p_-value vectors before log transformation) increased significantly following the regression-based correction (from r = 0.69 to r = 0.95). The spread around the diagonal (red line) also decreased following the regression correction (from a coefficient of variation (mean/std) of 1.13 to 0.56). The minimum
against the first four confounders listed in Table 1 (this approach does not require genotype data). The Pearson's correlation coefficient (calculated between _p_-value vectors before log transformation) increased significantly following the regression-based correction (from r = 0.69 to r = 0.95). The spread around the diagonal (red line) also decreased following the regression correction (from a coefficient of variation (mean/std) of 1.13 to 0.56). The minimum  is 10−4 as the _p_-values were calculated based on 1,000 permutations for genes with
is 10−4 as the _p_-values were calculated based on 1,000 permutations for genes with  , and 10,000 permutations for genes with
, and 10,000 permutations for genes with  . Some of the variation in the low _p_-value tail is due to having done only 10,000 permutations (
. Some of the variation in the low _p_-value tail is due to having done only 10,000 permutations ( ), and some to limitations of the linear regression method. Note that the four dots in (A) with
), and some to limitations of the linear regression method. Note that the four dots in (A) with  contain ten overlapping dots that refer to four sets of 2–3 genes, each set assigned the same
contain ten overlapping dots that refer to four sets of 2–3 genes, each set assigned the same  . Gene association _p_-values are plotted on a −log10(_p_-value) scale.
. Gene association _p_-values are plotted on a −log10(_p_-value) scale.
Figure 3. Estimating power of the GSEA algorithm in MAGENTA using computer simulations.
We used simulations to assess the power (sensitivity) of the gene set enrichment analysis (GSEA) algorithm in MAGENTA to detect enrichment of genes with modest effect sizes that are hard to detect with single SNP analysis. Power is plotted as a function of fraction (A) or number (B) of causal genes of modest effect in gene sets of 25 (triangles), 100 (squares), or 1,000 (circles) genes. The modest effect size spiked into genes is equivalent to 1% power of detecting an association at genome-wide significance using single SNP analysis. A total of 100 causal genes in the genome were assumed here. Randomized  vectors from case/control permutations of the DGI study were used as the background association values. Simulations were repeated 1,000 times for each unique set of parameters. Power was calculated as the fraction of times the simulated gene set received a
vectors from case/control permutations of the DGI study were used as the background association values. Simulations were repeated 1,000 times for each unique set of parameters. Power was calculated as the fraction of times the simulated gene set received a  <0.01. For specificity estimations we used SNPs with no effect size, sampled from a null distribution that assumes no association. The false positive rate of the method (1-specificity) was comparable to the _p_-value cutoff used (0.3–1.7%). Note the _x_-axis in both panels is on a log10 scale.
<0.01. For specificity estimations we used SNPs with no effect size, sampled from a null distribution that assumes no association. The false positive rate of the method (1-specificity) was comparable to the _p_-value cutoff used (0.3–1.7%). Note the _x_-axis in both panels is on a log10 scale.
Similar articles
- Gene set of nuclear-encoded mitochondrial regulators is enriched for common inherited variation in obesity.
Knoll N, Jarick I, Volckmar AL, Klingenspor M, Illig T, Grallert H, Gieger C, Wichmann HE, Peters A, Hebebrand J, Scherag A, Hinney A. Knoll N, et al. PLoS One. 2013;8(2):e55884. doi: 10.1371/journal.pone.0055884. Epub 2013 Feb 8. PLoS One. 2013. PMID: 23409076 Free PMC article. - Pathways targeted by antidiabetes drugs are enriched for multiple genes associated with type 2 diabetes risk.
Segrè AV, Wei N; DIAGRAM Consortium; MAGIC Investigators; Altshuler D, Florez JC. Segrè AV, et al. Diabetes. 2015 Apr;64(4):1470-83. doi: 10.2337/db14-0703. Epub 2014 Nov 3. Diabetes. 2015. PMID: 25368101 Free PMC article. - Common variation in oxidative phosphorylation genes is not a major cause of insulin resistance or type 2 diabetes.
Snogdal LS, Wod M, Grarup N, Vestmar M, Sparsø T, Jørgensen T, Lauritzen T, Beck-Nielsen H, Henriksen JE, Pedersen O, Hansen T, Højlund K. Snogdal LS, et al. Diabetologia. 2012 Feb;55(2):340-8. doi: 10.1007/s00125-011-2377-0. Epub 2011 Nov 18. Diabetologia. 2012. PMID: 22095239 - Novel biological insights emerging from genetic studies of type 2 diabetes and related metabolic traits.
De Silva NM, Frayling TM. De Silva NM, et al. Curr Opin Lipidol. 2010 Feb;21(1):44-50. doi: 10.1097/MOL.0b013e328334fdb6. Curr Opin Lipidol. 2010. PMID: 19956073 Review. - Transcribing β-cell mitochondria in health and disease.
Mulder H. Mulder H. Mol Metab. 2017 May 31;6(9):1040-1051. doi: 10.1016/j.molmet.2017.05.014. eCollection 2017 Sep. Mol Metab. 2017. PMID: 28951827 Free PMC article. Review.
Cited by
- The goldmine of GWAS summary statistics: a systematic review of methods and tools.
Kontou PI, Bagos PG. Kontou PI, et al. BioData Min. 2024 Sep 5;17(1):31. doi: 10.1186/s13040-024-00385-x. BioData Min. 2024. PMID: 39238044 Free PMC article. - Single-cell genomics improves the discovery of risk variants and genes of atrial fibrillation.
Selewa A, Luo K, Wasney M, Smith L, Sun X, Tang C, Eckart H, Moskowitz IP, Basu A, He X, Pott S. Selewa A, et al. Nat Commun. 2023 Aug 17;14(1):4999. doi: 10.1038/s41467-023-40505-5. Nat Commun. 2023. PMID: 37591828 Free PMC article. - Enlightening the molecular mechanisms of type 2 diabetes with a novel pathway clustering and pathway subnetwork approach.
Bakir-Gungor B, Ünlü Yazici M, Göy G, Temiz M. Bakir-Gungor B, et al. Turk J Biol. 2022 Jul 18;46(4):318-341. doi: 10.55730/1300-0152.2620. eCollection 2022. Turk J Biol. 2022. PMID: 37529091 Free PMC article. - Increased body mass index is linked to systemic inflammation through altered chromatin co-accessibility in human preadipocytes.
Garske KM, Kar A, Comenho C, Balliu B, Pan DZ, Bhagat YV, Rosenberg G, Koka A, Das SS, Miao Z, Sinsheimer JS, Kaprio J, Pietiläinen KH, Pajukanta P. Garske KM, et al. Nat Commun. 2023 Jul 14;14(1):4214. doi: 10.1038/s41467-023-39919-y. Nat Commun. 2023. PMID: 37452040 Free PMC article.
References
- Lowell BB, Shulman GI. Mitochondrial dysfunction and type 2 diabetes. Science. 2005;307:384–387. doi: 10.1126/science.1104343. - DOI - PubMed
- Dumas J, Simard G, Flamment M, Ducluzeau P, Ritz P. Is skeletal muscle mitochondrial dysfunction a cause or an indirect consequence of insulin resistance in humans? Diabetes Metab. 2009;35:159–167. doi: 10.1016/j.diabet.2009.02.002. - DOI - PubMed
- Taylor RW, Turnbull DM. Mitochondrial DNA mutations in human disease. Nat Rev Genet. 2005;6:389–402. doi: 10.1038/nrg1606. - DOI - PMC - PubMed
- Jin W, Patti M. Genetic determinants and molecular pathways in the pathogenesis of Type 2 diabetes. Clin Sci. 2009;116:99–111. doi: 10.1042/CS20080090. - DOI - PubMed
- Kelley DE, He J, Menshikova EV, Ritov VB. Dysfunction of mitochondria in human skeletal muscle in type 2 diabetes. Diabetes. 2002;51:2944–2950. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical