Anti-tumor activity of splice-switching oligonucleotides - PubMed (original) (raw)
Anti-tumor activity of splice-switching oligonucleotides
John A Bauman et al. Nucleic Acids Res. 2010 Dec.
Abstract
Alternative splicing has emerged as an important target for molecular therapies. Splice-switching oligonucleotides (SSOs) modulate alternative splicing by hybridizing to pre-mRNA sequences involved in splicing and blocking access to the transcript by splicing factors. Recently, the efficacy of SSOs has been established in various animal disease models; however, the application of SSOs against cancer targets has been hindered by poor in vivo delivery of antisense therapeutics to tumor cells. The apoptotic regulator Bcl-x is alternatively spliced to express anti-apoptotic Bcl-x(L) and pro-apoptotic Bcl-x(S). Bcl-x(L) is upregulated in many cancers and is associated with chemoresistance, distinguishing it as an important target for cancer therapy. We previously showed that redirection of Bcl-x pre-mRNA splicing from Bcl-x(L) to -x(S) induced apoptosis in breast and prostate cancer cells. In this study, the effect of SSO-induced Bcl-x splice-switching on metastatic melanoma was assessed in cell culture and B16F10 tumor xenografts. SSOs were delivered in vivo using lipid nanoparticles. Administration of nanoparticle with Bcl-x SSO resulted in modification of Bcl-x pre-mRNA splicing in lung metastases and reduced tumor load, while nanoparticle alone or formulated with a control SSO had no effect. Our findings demonstrate in vivo anti-tumor activity of SSOs that modulate Bcl-x pre-mRNA splicing.
Figures
Figure 1.
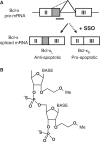
Direction of Bcl-x alternative splicing by SSO. (A) Use of the downstream or upstream alternative 5′-splice site within exon II of Bcl-x pre-mRNA yields anti-apoptotic Bcl-xL or pro-apoptotic Bcl-xS, respectively. SSO targeted to the downstream splice site redirects the splicing machinery to the upstream alternative splice site, resulting in a simultaneous decrease in production of Bcl-xL and increase in production of Bcl-xS. (B) Chemical structure of MOE phosphorothioate (PS) oligonucleotide. The MOE ribose modification confers RNase-H non-competence and increased affinity for target mRNA, while the PS inter-nucleotide linkage improves serum stability and bioavailability.
Figure 2.
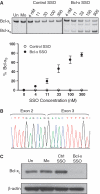
SSO induced Bcl-x splice-switching in B16F10 murine melanoma cells. (A) RT–PCR analysis of Bcl-x mRNA from cells transfected with control and Bcl-x SSO 24 h posttransfection. The Bcl-x SSO induces dose-dependant switching of Bcl-x mRNA splicing from anti-apoptotic Bcl-xL to pro-apoptotic Bcl-xS. Error bars indicate mean ± SD (n = 3). (B) Sequence analysis of the RT–PCR product corresponding to Bcl-xS showing exons 2 and 3 joined at the upstream alternative splice site. (C) Immunoblot analysis of cells treated with 100 nM Bcl-x SSO 48 h posttransfection. The SSO induces a reduction in the Bcl-xL protein isoform. Detection of β-actin confirms equal loading.
Figure 3.
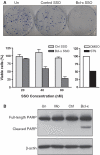
Bcl-x SSO induces cell death in B16F10 murine melanoma cells**.** (A) Effect of Bcl-x and control SSO on cell viability as determined by a clonogenic assay. Top panel shows images of representative plates at 80 nM concentration. Bottom left panel expresses viability of SSO-treated cells relative to untreated cells. Error bars indicate mean ± SD (n = 3–4). Bottom right panel shows viability of cells treated with 1 μM staurosporine (STS) relative to DMSO-treated cells. (B) Western blot of protein from cells treated with control and Bcl-x SSOs for 24 h. The presence of PARP cleavage in Bcl-x SSO-treated cells confirms that the observed decrease in cell viability is due to apoptosis. Detection of β-actin confirms equal loading.
Figure 4.
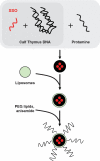
Schematic depiction of the preparation of SSO encapsulated LPD NP. The SSO was encapsulated into a condensed core with a high molecular weight polyanion (calf thymus DNA) and protamine, which was then coated with cationic liposomes. PEG lipids were inserted into the resultant bilayer to shield the positive charge and prevent aggregation in circulation. Anisamide, a commonly used targeting ligand that binds the sigma receptor, was conjugated to the distal end of the PEG lipids.
Figure 5.
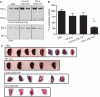
Effects of LPD-NP-delivered SSO in B16F10 tumor-bearing lungs. (A) RT–PCR analysis of Bcl-x mRNA splicing in the tumor-bearing lungs (upper panel) and liver (lower panel) of mice treated with vehicle only (PBS), empty NP, control SSO-formulated NP and Bcl-x SSO-formulated NP. (B) Luciferase activity in the tumor-loaded lungs on Day 17 after injections on Days 3–6. Asterisks denote statistical significance thresholds (*P < 0.05, **P < 0.001, determined by ANOVA and Tukey’s posttest). Untreated n = 26, NP only n = 8, Control SSO NP n = 13, Bcl-x SSO NP n = 9. (C) Representative images of lungs excised from tumor-bearing mice on Day 17 following four injections of 2.4 mg/kg NP formulations on Days 3–6.
Figure 6.
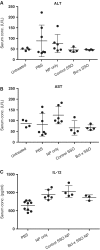
Serum enzyme and cytokine analysis. ALT (A), AST (B) and IL-12 (C) serum levels of untreated tumor-free and tumor-bearing C57BL/6 mice 24 h after the last of four consecutive daily treatments. Treatments consisted of i.v. injections of vehicle only (PBS), NP formulated with control SSO, or NP formulated with SSO targeted to Bcl-x.
Similar articles
- Pro-apoptotic effects of splice-switching oligonucleotides targeting Bcl-x pre-mRNA in human glioma cell lines.
Li Z, Li Q, Han L, Tian N, Liang Q, Li Y, Zhao X, Du C, Tian Y. Li Z, et al. Oncol Rep. 2016 Feb;35(2):1013-9. doi: 10.3892/or.2015.4465. Epub 2015 Nov 30. Oncol Rep. 2016. PMID: 26718027 - Modulation of RNA splicing as a potential treatment for cancer.
Bauman JA, Kole R. Bauman JA, et al. Bioeng Bugs. 2011 May-Jun;2(3):125-8. doi: 10.4161/bbug.2.3.15165. Epub 2011 May 1. Bioeng Bugs. 2011. PMID: 21637003 Free PMC article. - Modification of BCLX pre-mRNA splicing has antitumor efficacy alone or in combination with radiotherapy in human glioblastoma cells.
Dou Z, Lei H, Su W, Zhang T, Chen X, Yu B, Zhen X, Si J, Sun C, Zhang H, Di C. Dou Z, et al. Cell Death Dis. 2024 Feb 21;15(2):160. doi: 10.1038/s41419-024-06507-x. Cell Death Dis. 2024. PMID: 38383492 Free PMC article. - Development of therapeutic splice-switching oligonucleotides.
Disterer P, Kryczka A, Liu Y, Badi YE, Wong JJ, Owen JS, Khoo B. Disterer P, et al. Hum Gene Ther. 2014 Jul;25(7):587-98. doi: 10.1089/hum.2013.234. Epub 2014 Jun 19. Hum Gene Ther. 2014. PMID: 24826963 Free PMC article. Review. - Aberrant Bcl-x splicing in cancer: from molecular mechanism to therapeutic modulation.
Dou Z, Zhao D, Chen X, Xu C, Jin X, Zhang X, Wang Y, Xie X, Li Q, Di C, Zhang H. Dou Z, et al. J Exp Clin Cancer Res. 2021 Jun 12;40(1):194. doi: 10.1186/s13046-021-02001-w. J Exp Clin Cancer Res. 2021. PMID: 34118966 Free PMC article. Review.
Cited by
- The Impact of the Ca2+-Independent Phospholipase A2β (iPLA2β) on Immune Cells.
White TD, Almutairi A, Tusing YG, Lei X, Ramanadham S. White TD, et al. Biomolecules. 2021 Apr 15;11(4):577. doi: 10.3390/biom11040577. Biomolecules. 2021. PMID: 33920898 Free PMC article. Review. - Directing HER4 mRNA expression towards the CYT2 isoform by antisense oligonucleotide decreases growth of breast cancer cells in vitro and in vivo.
Nielsen TO, Sorensen S, Dagnæs-Hansen F, Kjems J, Sorensen BS. Nielsen TO, et al. Br J Cancer. 2013 Jun 11;108(11):2291-8. doi: 10.1038/bjc.2013.247. Epub 2013 May 21. Br J Cancer. 2013. PMID: 23695025 Free PMC article. - The Splicing Efficiency of Activating HRAS Mutations Can Determine Costello Syndrome Phenotype and Frequency in Cancer.
Hartung AM, Swensen J, Uriz IE, Lapin M, Kristjansdottir K, Petersen US, Bang JM, Guerra B, Andersen HS, Dobrowolski SF, Carey JC, Yu P, Vaughn C, Calhoun A, Larsen MR, Dyrskjøt L, Stevenson DA, Andresen BS. Hartung AM, et al. PLoS Genet. 2016 May 19;12(5):e1006039. doi: 10.1371/journal.pgen.1006039. eCollection 2016 May. PLoS Genet. 2016. PMID: 27195699 Free PMC article. - LncRNA CACClnc promotes chemoresistance of colorectal cancer by modulating alternative splicing of RAD51.
Zhang X, Ma D, Xuan B, Shi D, He J, Yu M, Xiong H, Ma Y, Shen C, Guo F, Cao Y, Yan Y, Gao Z, Tong T, Zhu X, Fang JY, Chen H, Hong J. Zhang X, et al. Oncogene. 2023 Apr;42(17):1374-1391. doi: 10.1038/s41388-023-02657-y. Epub 2023 Mar 11. Oncogene. 2023. PMID: 36906654 - Microsatellite Instability and Aberrant Pre-mRNA Splicing: How Intimate Is It?
Corcos L, Le Scanf E, Quéré G, Arzur D, Cueff G, Jossic-Corcos CL, Le Maréchal C. Corcos L, et al. Genes (Basel). 2023 Jan 25;14(2):311. doi: 10.3390/genes14020311. Genes (Basel). 2023. PMID: 36833239 Free PMC article.
References
- Lopez-Bigas N, Audit B, Ouzounis C, Parra G, Guigo R. Are splicing mutations the most frequent cause of hereditary disease? FEBS Lett. 2005;579:1900–1903. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials