Health status of adults with congenital adrenal hyperplasia: a cohort study of 203 patients - PubMed (original) (raw)
. 2010 Nov;95(11):5110-21.
doi: 10.1210/jc.2010-0917. Epub 2010 Aug 18.
Debbie S Willis, Sarah H Wild, Nils Krone, Emma J Doherty, Stefanie Hahner, Thang S Han, Paul V Carroll, Gerry S Conway, D Aled Rees, Roland H Stimson, Brian R Walker, John M C Connell, Richard J Ross; United Kingdom Congenital Adrenal Hyperplasia Adult Study Executive (CaHASE)
Collaborators, Affiliations
- PMID: 20719839
- PMCID: PMC3066446
- DOI: 10.1210/jc.2010-0917
Health status of adults with congenital adrenal hyperplasia: a cohort study of 203 patients
Wiebke Arlt et al. J Clin Endocrinol Metab. 2010 Nov.
Abstract
Context: No consensus exists for management of adults with congenital adrenal hyperplasia (CAH) due to a paucity of data from cohorts of meaningful size.
Objective: Our objective was to establish the health status of adults with CAH.
Design and setting: We conducted a prospective cross-sectional study of adults with CAH attending specialized endocrine centers across the United Kingdom.
Patients: Participants included 203 CAH patients (199 with 21-hydroxylase deficiency): 138 women, 65 men, median age 34 (range 18-69) years.
Main outcome measures: Anthropometric, metabolic, and subjective health status was evaluated. Anthropometric measurements were compared with Health Survey for England data, and psychometric data were compared with appropriate reference cohorts.
Results: Glucocorticoid treatment consisted of hydrocortisone (26%), prednisolone (43%), dexamethasone (19%), or a combination (10%), with reverse circadian administration in 41% of patients. Control of androgens was highly variable with a normal serum androstenedione found in only 36% of patients, whereas 38% had suppressed levels suggesting glucocorticoid overtreatment. In comparison with Health Survey for England participants, CAH patients were significantly shorter and had a higher body mass index, and women with classic CAH had increased diastolic blood pressure. Metabolic abnormalities were common, including obesity (41%), hypercholesterolemia (46%), insulin resistance (29%), osteopenia (40%), and osteoporosis (7%). Subjective health status was significantly impaired and fertility compromised.
Conclusions: Currently, a minority of adult United Kingdom CAH patients appear to be under endocrine specialist care. In the patients studied, glucocorticoid replacement was generally nonphysiological, and androgen levels were poorly controlled. This was associated with an adverse metabolic profile and impaired fertility and quality of life. Improvements in the clinical management of adults with CAH are required.
Trial registration: ClinicalTrials.gov NCT00749593.
Figures
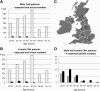
Figure 1
Expected and recruited number of CAH patients in the United Kingdom. Age distribution of recruited male (A) and female (B) CAH patients from 17 endocrine specialist centers across the United Kingdom (C) in comparison with the expected number of classic CAH patients based on United Kingdom population data (Office of National Statistics) and an assumed incidence of one in 12,000 newborns. A and B show absolute numbers (white bars, normal population; black bars, CAH patients); D shows percentage of the expected patient number per age group (gray bars, males; black bars, females).
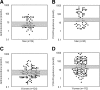
Figure 2
Serum levels of the androgen precursor androstenedione (A, C) and the glucocorticoid precursor 17OHP (B, D) in male and female CAH patients sampled in the morning after intake of the usual glucocorticoid morning dose. The shaded areas represent recommended target range (2). Note the logarithmic scale used for representing the serum hormone concentrations.
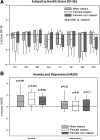
Figure 3
Subjective health in status in CAH according to SF-36 (A) and HADS (B) questionnaires and shown for male classic (n = 65), female classic (n = 103), and female nonclassic (n = 31) CAH patients with box plots representing median and interquartile ranges; whiskers represent the 5th and 95th percentiles. A z-score of 0 represents the median of the normal reference population; the lower the SF-36 z-scores and the higher the HADS z-scores, the worse is self-perceived wellbeing and mood. SF-36 dimensions: PF, physical functioning; RP, role limitations due to physical problems; BP, bodily pain; GH, general health; VT, vitality; SF, social functioning; RE, role limitations due to emotional problems; MH, mental health.
Similar articles
- Glucocorticoid replacement regimens for treating congenital adrenal hyperplasia.
Ng SM, Stepien KM, Krishan A. Ng SM, et al. Cochrane Database Syst Rev. 2020 Mar 19;3(3):CD012517. doi: 10.1002/14651858.CD012517.pub2. Cochrane Database Syst Rev. 2020. PMID: 32190901 Free PMC article. - Quality of life in adults with congenital adrenal hyperplasia relates to glucocorticoid treatment, adiposity and insulin resistance: United Kingdom Congenital adrenal Hyperplasia Adult Study Executive (CaHASE).
Han TS, Krone N, Willis DS, Conway GS, Hahner S, Rees DA, Stimson RH, Walker BR, Arlt W, Ross RJ; United Kingdom Congenital adrenal Hyperplasia Adult Study Executive (CaHASE). Han TS, et al. Eur J Endocrinol. 2013 May 3;168(6):887-93. doi: 10.1530/EJE-13-0128. Print 2013 Jun. Eur J Endocrinol. 2013. PMID: 23520247 Free PMC article. - Obesity among children and adolescents with classic congenital adrenal hyperplasia due to 21-hydroxylase deficiency.
Völkl TM, Simm D, Beier C, Dörr HG. Völkl TM, et al. Pediatrics. 2006 Jan;117(1):e98-105. doi: 10.1542/peds.2005-1005. Pediatrics. 2006. PMID: 16396852 - Cardio-metabolic risk factors in youth with classical 21-hydroxylase deficiency.
Ariyawatkul K, Tepmongkol S, Aroonparkmongkol S, Sahakitrungruang T. Ariyawatkul K, et al. Eur J Pediatr. 2017 Apr;176(4):537-545. doi: 10.1007/s00431-017-2875-2. Epub 2017 Feb 21. Eur J Pediatr. 2017. PMID: 28224294 - Treatment and health outcomes in adults with congenital adrenal hyperplasia.
Han TS, Walker BR, Arlt W, Ross RJ. Han TS, et al. Nat Rev Endocrinol. 2014 Feb;10(2):115-24. doi: 10.1038/nrendo.2013.239. Epub 2013 Dec 17. Nat Rev Endocrinol. 2014. PMID: 24342885 Review.
Cited by
- Novel treatment strategies in congenital adrenal hyperplasia.
Turcu AF, Auchus RJ. Turcu AF, et al. Curr Opin Endocrinol Diabetes Obes. 2016 Jun;23(3):225-32. doi: 10.1097/MED.0000000000000256. Curr Opin Endocrinol Diabetes Obes. 2016. PMID: 27032061 Free PMC article. Review. - Increased Abdominal Adiposity in Adolescents and Young Adults With Classical Congenital Adrenal Hyperplasia due to 21-Hydroxylase Deficiency.
Kim MS, Ryabets-Lienhard A, Dao-Tran A, Mittelman SD, Gilsanz V, Schrager SM, Geffner ME. Kim MS, et al. J Clin Endocrinol Metab. 2015 Aug;100(8):E1153-9. doi: 10.1210/jc.2014-4033. Epub 2015 Jun 10. J Clin Endocrinol Metab. 2015. PMID: 26062016 Free PMC article. - Nonclassic congenital adrenal hyperplasia due to 21-hydroxylase deficiency: clinical presentation, diagnosis, treatment, and outcome.
Falhammar H, Nordenström A. Falhammar H, et al. Endocrine. 2015 Sep;50(1):32-50. doi: 10.1007/s12020-015-0656-0. Epub 2015 Jun 17. Endocrine. 2015. PMID: 26082286 Review. - Congenital adrenal hyperplasia and pregnancy.
Shorakae S, Teede H. Shorakae S, et al. BMJ Case Rep. 2013 Aug 5;2013:bcr2013010299. doi: 10.1136/bcr-2013-010299. BMJ Case Rep. 2013. PMID: 23917362 Free PMC article. - Interpretation of Steroid Biomarkers in 21-Hydroxylase Deficiency and Their Use in Disease Management.
Sarafoglou K, Merke DP, Reisch N, Claahsen-van der Grinten H, Falhammar H, Auchus RJ. Sarafoglou K, et al. J Clin Endocrinol Metab. 2023 Aug 18;108(9):2154-2175. doi: 10.1210/clinem/dgad134. J Clin Endocrinol Metab. 2023. PMID: 36950738 Free PMC article. Review.
References
- Arlt W, Allolio B 2003 Adrenal insufficiency. Lancet 361:1881–1893 - PubMed
- Merke DP, Bornstein SR 2005 Congenital adrenal hyperplasia. Lancet 365:2125–2136 - PubMed
- Speiser PW, White PC 2003 Congenital adrenal hyperplasia. N Engl J Med 349:776–788 - PubMed
- Arlt W, Krone N 2007 Adult consequences of congenital adrenal hyperplasia. Horm Res 68(Suppl 5):158–164 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- WT_/Wellcome Trust/United Kingdom
- G0400874/MRC_/Medical Research Council/United Kingdom
- G0900567/MRC_/Medical Research Council/United Kingdom
- GR079865MA/WT_/Wellcome Trust/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical