Structural dynamics of synapses in vivo correlate with functional changes during experience-dependent plasticity in visual cortex - PubMed (original) (raw)
Structural dynamics of synapses in vivo correlate with functional changes during experience-dependent plasticity in visual cortex
Daniela Tropea et al. J Neurosci. 2010.
Abstract
The impact of activity on neuronal circuitry is complex, involving both functional and structural changes whose interaction is largely unknown. We have used optical imaging of mouse visual cortex responses and two-photon imaging of superficial layer spines on layer 5 neurons to monitor network function and synaptic structural dynamics in the mouse visual cortex in vivo. Total lack of vision due to dark-rearing from birth dampens visual responses and shifts spine dynamics and morphologies toward an immature state. The effects of vision after dark rearing are strongly dependent on the timing of exposure: over a period of days, functional and structural changes are temporally related such that light stabilizes spines while increasing visually driven activity. The effects of long-term light exposure can be partially mimicked by experimentally enhancing inhibitory signaling in the darkness. Brief light exposure, however, results in a rapid, transient, NMDA-dependent increase of cortical responses, accompanied by increased dynamics of dendritic spines. These findings indicate that visual experience induces rapid reorganization of cortical circuitry followed by a period of stabilization, and demonstrate a close relationship between dynamic changes at single synapses and cortical network function.
Figures
Figure 1.
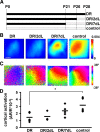
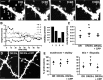
Visual experience regulates cortical responses to vision. A, Time course of the experiment: all animals were imaged at P28. The period of dark rearing is represented as a black line, while the white-dotted pattern represents the period of exposure to a normal light environment. DR animals were maintained and anesthetized before imaging in complete darkness. DR/2 dl animals were dark-reared until P26 and then exposed to light. DR/7 dl animals were dark-reared until P21 and then exposed to light. Control animals were reared in a normal 12 h light/12 h dark environment. B, Representative images of the cortical intrinsic signal in response to a visual stimulus in individual mice from the different groups imaged. Red hues indicate strong activation, according to color key at right depicting the change in reflectance, dR/R. In dark-reared mice, cortical activity in response to light is low and increases as mice are exposed to light. Scale bar, 0.5 mm. C, Representative retinotopic maps of visual field elevation in individual mice with different visual experience. The map is highly disorganized in dark-reared animals and the level of organization progressively increases together with the duration of light exposure. Color key at right depicts visual elevation. D, Quantification of the visually evoked optical signal across animals with different visual experience. The strength of the signal (normalized change in reflectance, dR/R) is low in dark-reared animals and recovers with longer light exposure. *p < 0.05 when compared with control.
Figure 2.
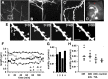
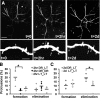
Visual experience regulates dendritic spine structure in the visual cortex. A, In vivo two-photon image of the apical tuft of a layer 5 pyramidal neuron in visual cortex of a P28 mouse. The image is a collapsed z stack showing the extent of the apical tuft dendritic arbor. Scale bar, 100 μm. B, Three-dimensional reconstruction showing the dendritic arbor from the side. Scale bar, 100 μm. C, Two-photon image of dendritic spines in vivo from dendrite shown in A (boxed area). Scale bar, 5 μm. D, Following imaging, red tracer was injected into the imaged area based on the blood vessel pattern. The animal was perfused and the imaged area identified in fixed section. The injection site is shown in red and resides in visual cortex as identified using coordinates of the mouse brain atlas. E, Time-lapse image of dendritic spines in the visual cortex of a control mouse in vivo. Images shown were taken 30 min apart. Scale bar, 2 μm. Spines are motile at these ages (notice spine 3 withdraws into the dendrite during the first hour of imaging). F, Lengths of the 4 spines at left are shown plotted over 2 h. G, The motility index for the same four spines showing the approximate range of motilities observed in control animals (filopodia are not shown in this figure—filopodia were generally more motile and were rarely observed in control animals). H, Spine motility indices for P28 mice exposed to different visual environments. Analysis included all spine classes and filopodia. There is a significant increase in spine motility in visually deprived animals (DR) compared with controls. Two days of exposure to a normal light-dark cycle does not affect motility but after 7 d of exposure to normal dark-light conditions the motility index is no longer significantly different from that in control animals. *p < 0.05 compared with control.
Figure 3.
Visual experience affects spine morphology in visual cortex. A, High-magnification two-photon images of representative dendrites in the visual cortex of animals reared in different visual environments. Scale bar, 5 μm. B, Image showing the classification of dendritic spines into morphological classes. M, mushroom; S, stubby; T, thin; F, filopodium. C, Variations in the morphologies of dendritic spines across animals with different visual experience. Animals reared in normal conditions show a significantly higher percentage of mushroom and stubby spines compared with animals that have been visually deprived, which show increased numbers of thin spines and filopodia (*p < 0.05, comparing control to other conditions). Seven days of reexposure to light after dark-rearing restores the morphological profile of dendritic spines to control levels.
Figure 4.
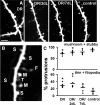
Increased inhibitory drive causes functional and structural reorganization in dark-reared visual cortex. A, Time course of the experiment: all animals were imaged at P28. Five groups of animals were used: DR animals were maintained and anesthetized before imaging in complete darkness; DR/7 dl animals were dark-reared until P21 and then exposed to light; DR/7dDIA animals were maintained in the darkness but injected with a daily dose of diazepam starting at P21; control animals were reared in a normal 12 h light/12 h dark environment. B, Representative images of the cortical intrinsic signal in response to light in individual mice from the different groups imaged. Red hues indicate strong activation, according to the dR/R scale at right. Mice treated with diazepam (DR/7dDIA) show strong visually driven responses similar to control mice and dark-reared mice exposed to light for 7 d (DR/7 dl). Scale bar, 0.5 mm. C, Representative retinotopic maps of elevation in individual mice, as per key at right. Diazepam treatment results in an increase in the level of organization of the visual map similar to light exposure. Scale bar, 0.5 mm. D, Quantification of the visually evoked optical signal across animals with different visual experience. The strength of the signal (normalized change in reflectance, dR/R) is low in dark-reared animals and increases in animals exposed to light for 7 d as well as in animals treated in the dark with diazepam (n = 3 animals). Only animals in the DR group had ΔR/R values that were significantly different from control animals (*p < 0.05). E, Image showing a dendritic branch in an animal treated with diazepam (DR/7dDIA). Scale bar, 5 μm. F, Diazepam treatment reduced the motility of spines in dark-reared animals (n = 7 animals) to control levels. G, Spine morphology was not affected by diazepam treatment and these animals had increased numbers of thin spines and filopodia when compared with control animals (*p < 0.05).
Figure 5.
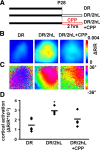
Light exposure induces rapid functional reorganization mediated by NMDA receptors. A, Schematic of experimental timeline. Three groups of animals were used: animals reared in the darkness from birth (DR; same as Fig. 1, 3,4), DR animals exposed to light for 2 h before imaging (DR/2hL) and DR animals in which the NMDA antagonist CPP was injected systemically 30 min before 2 h of light exposure (DR/2hL+CPP). B, Representative images of the cortical intrinsic signal in response to light in individual mice from the different groups imaged. Red hues indicate strong activation, as per dR/R scale at right. Mice exposed to light for 2 h show strong visually driven responses similar to control mice. Mice pretreated with the NMDA receptor antagonist CPP do not show increased visual responses after brief light exposure. Scale bar, 0.5 mm. C, Representative retinotopic maps of elevation in individual mice, as per key at right. D, Quantification of the amplitude of visually evoked cortical activation in the three groups of animals. Brief light exposure significantly increased visually evoked cortical activation (*p < 0.05 compared with dark-reared animals). This effect was prevented by systemic injection of CPP before light exposure.
Figure 6.
Light exposure induces rapid structural reorganization mediated by NMDA receptors. A, Time-lapse image of dendritic spines in the visual cortex of a dark-reared mouse in vivo following light exposure for 2 h at P28. Images shown were taken 20 min apart. Scale bar, 2 μm. Dendritic protrusions are highly motile in this group of animals (DR/2hL). B, Lengths of the 4 protrusions labeled in A are shown in the left panel plotted over 2 h. Notice the large changes observed in length over this timescale. The right panel shows the motility index for the same four protrusions. Protrusion 1 was classified as a filopodium due to its length. Not all spines are highly motile. Spine 3 exhibits length changes and a motility index typical of spines in control mice at this age. C, Brief light exposure rapidly increases spine motility in dark-reared animals (*p < 0.05). This effect is prevented when CPP is administered systemically before light exposure. D, High-magnification two-photon images of representative dendrites in the visual cortex of animals exposed to light for 2 h following dark rearing. Notice the increased numbers of thin spines and filopodia in dark-reared animals briefly exposed to light. Animals pretreated with CPP before light exposure have fewer thin spines and filopodia than untreated animals, but similar in proportion to dark-reared animals. Scale bar, 5 μm. E, Brief light exposure increases the proportion of thin protrusions (thin spines and filopodia) and decreases the proportion of mushroom and stubby spines compared with DR animals (*p < 0.05). The reorganization of dendritic spine morphology is prevented by administration of CPP before light exposure.
Figure 7.
Light exposure induces a rapid, transient outgrowth of dendritic protrusions. A, Top, A dendritic arbor imaged over 2 d in a dark-reared animal exposed to light after the first imaging session. Bottom, A higher magnification of a dendrite bearing dendritic spines. Notice the outgrowth of a filopodium (arrowhead) and spine (arrow). The filopodium withdraws after 2 d of light exposure but the spine is maintained. Scale bar, 30 μm (top); 5 μm (bottom). B, The formation of dendritic protrusions is enhanced after 2 h of light exposure in dark-reared animals relative to animals maintained in the dark and relative to normal light-reared controls (*p < 0.05), while the rate of elimination is not altered. C, The percentage of new protrusions formed between the end of the dark rearing period and after 2 d of light exposure is not different from that over a 2 d period in light-reared animals. However, the elimination of spines is significantly enhanced suggesting a pruning of preexisting synapses.
Similar articles
- Maternal Loss of Ube3a Impairs Experience-Driven Dendritic Spine Maintenance in the Developing Visual Cortex.
Kim H, Kunz PA, Mooney R, Philpot BD, Smith SL. Kim H, et al. J Neurosci. 2016 Apr 27;36(17):4888-94. doi: 10.1523/JNEUROSCI.4204-15.2016. J Neurosci. 2016. PMID: 27122043 Free PMC article. - Rapid experience-dependent plasticity of synapse function and structure in ferret visual cortex in vivo.
Yu H, Majewska AK, Sur M. Yu H, et al. Proc Natl Acad Sci U S A. 2011 Dec 27;108(52):21235-40. doi: 10.1073/pnas.1108270109. Epub 2011 Dec 12. Proc Natl Acad Sci U S A. 2011. PMID: 22160713 Free PMC article. - Stimulus-dependent synaptic plasticity underlies neuronal circuitry refinement in the mouse primary visual cortex.
Lopez-Ortega E, Choi JY, Hong I, Roth RH, Cudmore RH, Huganir RL. Lopez-Ortega E, et al. Cell Rep. 2024 Apr 23;43(4):113966. doi: 10.1016/j.celrep.2024.113966. Epub 2024 Mar 19. Cell Rep. 2024. PMID: 38507408 Free PMC article. - Dendritic spine plasticity--current understanding from in vivo studies.
Knott G, Holtmaat A. Knott G, et al. Brain Res Rev. 2008 Aug;58(2):282-9. doi: 10.1016/j.brainresrev.2008.01.002. Epub 2008 Mar 19. Brain Res Rev. 2008. PMID: 18353441 Review. - Use-dependent inhibition of dendritic spines.
Keller A. Keller A. Trends Neurosci. 2002 Nov;25(11):541-3; discussion 543-4. doi: 10.1016/s0166-2236(02)02260-9. Trends Neurosci. 2002. PMID: 12392919 Free PMC article. Review.
Cited by
- Phase-specific plasticity of synaptic structures in the somatosensory cortex of living mice during neuropathic pain.
Kim SK, Kato G, Ishikawa T, Nabekura J. Kim SK, et al. Mol Pain. 2011 Nov 9;7:87. doi: 10.1186/1744-8069-7-87. Mol Pain. 2011. PMID: 22067412 Free PMC article. - Control of Dendritic Spine Morphological and Functional Plasticity by Small GTPases.
Woolfrey KM, Srivastava DP. Woolfrey KM, et al. Neural Plast. 2016;2016:3025948. doi: 10.1155/2016/3025948. Epub 2016 Feb 18. Neural Plast. 2016. PMID: 26989514 Free PMC article. Review. - Juvenile depletion of microglia reduces orientation but not high spatial frequency selectivity in mouse V1.
Velez DXF, Arreola M, Huh CYL, Green K, Gandhi SP. Velez DXF, et al. Sci Rep. 2022 Jul 27;12(1):12779. doi: 10.1038/s41598-022-15503-0. Sci Rep. 2022. PMID: 35896554 Free PMC article. - The "quad-partite" synapse: microglia-synapse interactions in the developing and mature CNS.
Schafer DP, Lehrman EK, Stevens B. Schafer DP, et al. Glia. 2013 Jan;61(1):24-36. doi: 10.1002/glia.22389. Epub 2012 Jul 24. Glia. 2013. PMID: 22829357 Free PMC article. Review. - Spatiotemporal dynamics of dendritic spines in the living brain.
Chen CC, Lu J, Zuo Y. Chen CC, et al. Front Neuroanat. 2014 May 9;8:28. doi: 10.3389/fnana.2014.00028. eCollection 2014. Front Neuroanat. 2014. PMID: 24847214 Free PMC article. Review.
References
- Berardi N, Pizzorusso T, Ratto GM, Maffei L. Molecular basis of plasticity in the visual cortex. Trends Neurosci. 2003;26:369–378. - PubMed
- Bonhoeffer T, Yuste R. Spine motility. Phenomenology, mechanisms, and function. Neuron. 2002;35:1019–1027. - PubMed
- Brakeman PR, Lanahan AA, O'Brien R, Roche K, Barnes CA, Huganir RL, Worley PF. Homer: a protein that selectively binds metabotropic glutamate receptors. Nature. 1997;386:284–288. - PubMed
- Buisseret P, Gary-Bobo E, Imbert M. Plasticity in the kitten's visual cortex: effects of the suppression of visual experience upon the orientational properties of visual cortical cells. Brain Res. 1982;256:417–426. - PubMed
- Chen L, Yang C, Mower GD. Developmental changes in the expression of GABA(A) receptor subunits (alpha(1), alpha(2), alpha(3)) in the cat visual cortex and the effects of dark rearing. Brain Res Mol Brain Res. 2001;88:135–143. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- F32 EY007023/EY/NEI NIH HHS/United States
- R01 EY019277/EY/NEI NIH HHS/United States
- R01 EY007023/EY/NEI NIH HHS/United States
- EY007023/EY/NEI NIH HHS/United States
- 1F32EY017240/EY/NEI NIH HHS/United States
- WT_/Wellcome Trust/United Kingdom
- R01 EY017098/EY/NEI NIH HHS/United States
- EY017098/EY/NEI NIH HHS/United States
- F32 EY017240/EY/NEI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases