A noncompetitive BACE1 inhibitor TAK-070 ameliorates Abeta pathology and behavioral deficits in a mouse model of Alzheimer's disease - PubMed (original) (raw)
. 2010 Aug 18;30(33):11157-66.
doi: 10.1523/JNEUROSCI.2884-10.2010.
Hideki Takahashi, Naoki Tarui, Junji Matsui, Taisuke Tomita, Mitsuhiro Hirode, Masumi Sagayama, Ryouta Maeda, Makiko Kawamoto, Kazuko Hirai, Jun Terauchi, Yasufumi Sakura, Mitsuru Kakihana, Kaneyoshi Kato, Takeshi Iwatsubo, Masaomi Miyamoto
Affiliations
- PMID: 20720123
- PMCID: PMC6633483
- DOI: 10.1523/JNEUROSCI.2884-10.2010
A noncompetitive BACE1 inhibitor TAK-070 ameliorates Abeta pathology and behavioral deficits in a mouse model of Alzheimer's disease
Hiroaki Fukumoto et al. J Neurosci. 2010.
Abstract
We discovered a nonpeptidic compound, TAK-070, that inhibited BACE1, a rate-limiting protease for the generation of Abeta peptides that are considered causative for Alzheimer's disease (AD), in a noncompetitive manner. TAK-070 bound to full-length BACE1, but not to truncated BACE1 lacking the transmembrane domain. Short-term oral administration of TAK-070 decreased the brain levels of soluble Abeta, increased that of neurotrophic sAPPalpha by approximately 20%, and normalized the behavioral impairments in cognitive tests in Tg2576 mice, an APP transgenic mouse model of AD. Six-month chronic treatment decreased cerebral Abeta deposition by approximately 60%, preserving the pharmacological efficacy on soluble Abeta and sAPPalpha levels. These results support the feasibility of BACE1 inhibition with a noncompetitive inhibitor as disease-modifying as well as symptomatic therapy for AD.
Figures
Figure 1.
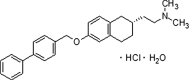
Chemical structure of TAK-070.
Figure 2.
Effects of TAK-070 on secretion of Aβ and sAPPα in cultured cells. The levels of Aβ40, Aβ42, and sAPPα secreted in conditioned media were quantitated by ELISAs. A, Human IMR32 neuroblastoma cells were treated with TAK-070 for 24 h. Vehicle control levels for Aβ40 and Aβ42 were 17.3 and 5.8 fmol/ml on average, respectively. Levels of sAPPα were determined as arbitrary unit values. Values are mean percentages relative to levels in the control (±SEM) in four independent experiments. B, Mouse Neuro2a neuroblastoma cells stably expressing human APPsw (N2aAPPsw cells) were treated with TAK-070 for 24 h. Vehicle control levels of Aβ40 and Aβ42 were 447.6 and 114.6 fmol/ml, respectively. Levels of sAPPα were determined as arbitrary unit values. Values are mean percentages of the control (±SEM) in six independent experiments. *p < 0.025, compared with the vehicle control (one-tailed Williams' test).
Figure 3.
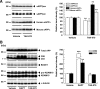
Immunoblot analysis of the protein levels of APP derivatives and those of secretases in N2aAPPsw cells. A, Immunoblots of sAPPβ (sAPPβsw derived from transfected human APPsw and sAPPβwt from endogenous mouse APP) and sAPPα (human sAPPα derived from transfected human APPsw and mouse sAPPα from endogenous APP) in media after treatment with TAK-070 (3 μmol/L) or vehicle from three independent experiments are shown. Values in the graph (right) show the mean percentages of band intensities analyzed by densitometry relative to those in vehicle control (±SEM) in the three independent experiments. *p < 0.05, **p < 0.01, versus vehicle control (Student's t test). B, Immunoblots of APP, C-terminal fragments of APP (C99 and C83), BACE1 and high- and low-molecular-weight forms of ADAM10 (pro- and matured forms, respectively) from lysates of N2aAPPsw cells treated with vehicle, DAPT (3 μmol/L), or TAK-070 (3 μmol/L) are shown. Levels of α-tubulin are shown as an internal control. Note that all the immunoblot data are obtained from a single membrane replica with identical exposure. Values for C99 and C83 (right) are mean percentages of band intensities analyzed by densitometry relative to those in vehicle control (±SEM) in the three independent experiments. *p < 0.05, **p < 0.01, ***p < 0.001, versus vehicle control (Student's t test).
Figure 4.
Noncompetitive inhibition of BACE1 activity by TAK-070 in cell-free assay. A, Concentration-dependent inhibition of BACE1 activity by TAK-070. Human recombinant full-length BACE1 purified from COS-7 cells (rhBACE1) was incubated with a fluorogenic BACE1 substrate based on the amino acid sequence of wild-type human APP flanking the BACE1 cleavage site (Nma-SEVKMDAEK(Dnp)RR-NH2) in the presence of various concentrations of TAK-070 (indicated in abscissa, in nanomoles per liter). Values are mean percentage inhibition (±SEM) in three independent experiments. B, Lineweaver–Burk plot analysis of the mode of inhibition by TAK-070. rhBACE1 was incubated with 20–200 μmol/L BACE1 substrate in the presence (10 or 30 μmol/L) or absence of TAK-070. The plots of 1/V versus 1/[S] were fitted by the Lineweaver–Burk straight line. Result of a representative experiment is shown.
Figure 5.
Surface plasmon resonance assay of the binding of TAK-070 to BACE1. A, Sensorgram showing a binding of TAK-070 to full-length BACE1 (1-501) (left panel), but not to C-terminally truncated BACE1 (1-454) lacking the membrane spanning region (right panel). TAK-070 bound to full-length BACE1 in a concentration-dependent manner (within the range of 0.5–8 μmol/L). B, Binding of TAK-070 (5 and 10 μmol/L) to full-length BACE1 (1-501) or C-terminally truncated BACE1 (1-474), (1-471), (1-465), and (1-460). One relative unit (RU) corresponds to 1 pg/mm2.
Figure 6.
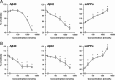
Effects of TAK-070 on Aβ and sAPPα levels in the brains of Tg2576 mice. A, Levels of Tris-soluble Aβ40, Aβ42, and sAPPα in the cerebral cortices of young female Tg2576 mice after short-term administration. Values are mean percentages (±SEM) relative to levels in vehicle control (n = 15 for both cohorts). *p < 0.025, versus vehicle control (one-tailed Williams test). B, Levels of Tris-soluble Aβ40, Aβ42, and sAPPα in cerebral cortices of 13-month-old Tg2576 mice after long-term treatment. The number of 13-month-old Tg2576 mice with vehicle or TAK-070 (56 ppm, corresponding to ∼7 mg/kg/d, p.o.) were 13 (male 6, female 7) and 16 (male 10, female 6), respectively after 6 months treatment. Values are mean percentages (±SEM) relative to levels in young controls (8-month-old nontreated Tg2576, n = 9). C, Levels of Tris-insoluble, formic acid-extractable Aβ40 and Aβ42 in cerebral cortices examined in B. Values are the fold increase (±SEM) relative to levels in young controls (8-month-old nontreated Tg2576). D, Aβ immunohistochemistry of coronal sections from brains of TAK-070 (bottom panel) or vehicle (top panel) treated-Tg2576 mice (13 months old). E, Amyloid burden (% of area covered by Aβ immunoreactivity; left panel) or density of plaque (number per mm2 area; right panel) in the cerebral neocortices of Tg2576 mice. Mean values (±SEM) are shown. ##p < 0.01, versus those in 8-month-old mice; *p < 0.05, **p < 0.01, versus those in vehicle control (Student's t test) in B, C, and E.
Figure 7.
Effects of TAK-070 on impaired behavior of Tg2576 mice in Y-maze test and Morris water maze test. A, Spontaneous alternations (as a percentage) in Y-maze test. B, C, Escape latency (in seconds) (B) and swimming distance (in centimeters) (C) of mice in the invisible Morris water maze test. Male Tg2576 mice (18 weeks old) were treated with TAK-070 (1 or 3 mg/kg, p.o.) or vehicle for 9 d and then sequentially tested in Y-maze on day 10 and Morris water maze tests on days 11–13. Mean values (±SEM) in 14 animals in each Tg2576 mice group and in 15 wild-type mice (Wild) are shown. *p < 0.05, **p < 0.01, versus those in Wild (Student's t test); +p < 0.025, versus those in the vehicle-treated Tg2576 mice (Williams' test).
Figure 8.
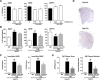
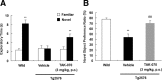
Effects of TAK-070 on impaired behavior of Tg2576 mice in a novel object recognition test. Mean (±SEM) time spent interacting with familiar or novel objects (A) and the novel object preference ratio (±SEM) (B) in the retention test conducted 24 h after the acquisition trial are shown. A, **p < 0.01, versus the familiar control object. B, **p < 0.01, versus the wild control, ##p < 0.01, versus the vehicle-treated control (Student's t test).
Similar articles
- Centrally Delivered BACE1 Inhibitor Activates Microglia, and Reverses Amyloid Pathology and Cognitive Deficit in Aged Tg2576 Mice.
Thakker DR, Sankaranarayanan S, Weatherspoon MR, Harrison J, Pierdomenico M, Heisel JM, Thompson LA, Haskell R, Grace JE, Taylor SJ, Albright CF, Shafer LL. Thakker DR, et al. J Neurosci. 2015 Apr 29;35(17):6931-6. doi: 10.1523/JNEUROSCI.2262-14.2015. J Neurosci. 2015. PMID: 25926467 Free PMC article. - BACE1 inhibition more effectively suppresses initiation than progression of β-amyloid pathology.
Peters F, Salihoglu H, Rodrigues E, Herzog E, Blume T, Filser S, Dorostkar M, Shimshek DR, Brose N, Neumann U, Herms J. Peters F, et al. Acta Neuropathol. 2018 May;135(5):695-710. doi: 10.1007/s00401-017-1804-9. Epub 2018 Jan 11. Acta Neuropathol. 2018. PMID: 29327084 Free PMC article. - AZ-4217: a high potency BACE inhibitor displaying acute central efficacy in different in vivo models and reduced amyloid deposition in Tg2576 mice.
Eketjäll S, Janson J, Jeppsson F, Svanhagen A, Kolmodin K, Gustavsson S, Radesäter AC, Eliason K, Briem S, Appelkvist P, Niva C, Berg AL, Karlström S, Swahn BM, Fälting J. Eketjäll S, et al. J Neurosci. 2013 Jun 12;33(24):10075-84. doi: 10.1523/JNEUROSCI.1165-13.2013. J Neurosci. 2013. PMID: 23761903 Free PMC article. - Beta-secretase inhibitors in phase I and phase II clinical trials for Alzheimer's disease.
Moussa CE. Moussa CE. Expert Opin Investig Drugs. 2017 Oct;26(10):1131-1136. doi: 10.1080/13543784.2017.1369527. Epub 2017 Aug 23. Expert Opin Investig Drugs. 2017. PMID: 28817311 Review. - BACE1 in Alzheimer's disease.
Sathya M, Premkumar P, Karthick C, Moorthi P, Jayachandran KS, Anusuyadevi M. Sathya M, et al. Clin Chim Acta. 2012 Dec 24;414:171-8. doi: 10.1016/j.cca.2012.08.013. Epub 2012 Aug 20. Clin Chim Acta. 2012. PMID: 22926063 Review.
Cited by
- Longitudinal testing of hippocampal plasticity reveals the onset and maintenance of endogenous human Aß-induced synaptic dysfunction in individual freely behaving pre-plaque transgenic rats: rapid reversal by anti-Aß agents.
Qi Y, Klyubin I, Harney SC, Hu N, Cullen WK, Grant MK, Steffen J, Wilson EN, Do Carmo S, Remy S, Fuhrmann M, Ashe KH, Cuello AC, Rowan MJ. Qi Y, et al. Acta Neuropathol Commun. 2014 Dec 24;2:175. doi: 10.1186/s40478-014-0175-x. Acta Neuropathol Commun. 2014. PMID: 25540024 Free PMC article. - Generation of gene-targeted mice using embryonic stem cells derived from a transgenic mouse model of Alzheimer's disease.
Yamamoto S, Ooshima Y, Nakata M, Yano T, Matsuoka K, Watanabe S, Maeda R, Takahashi H, Takeyama M, Matsumoto Y, Hashimoto T. Yamamoto S, et al. Transgenic Res. 2013 Jun;22(3):537-47. doi: 10.1007/s11248-012-9651-x. Epub 2012 Sep 9. Transgenic Res. 2013. PMID: 22961199 - A peptide binding to the β-site of APP improves spatial memory and attenuates Aβ burden in Alzheimer's disease transgenic mice.
Yang SG, Wang SW, Zhao M, Zhang R, Zhou WW, Li YN, Su YJ, Zhang H, Yu XL, Liu RT. Yang SG, et al. PLoS One. 2012;7(11):e48540. doi: 10.1371/journal.pone.0048540. Epub 2012 Nov 1. PLoS One. 2012. PMID: 23133641 Free PMC article. - Critical analysis of the use of β-site amyloid precursor protein-cleaving enzyme 1 inhibitors in the treatment of Alzheimer's disease.
Evin G, Barakat A. Evin G, et al. Degener Neurol Neuromuscul Dis. 2014 Jan 22;4:1-19. doi: 10.2147/DNND.S41056. eCollection 2014. Degener Neurol Neuromuscul Dis. 2014. PMID: 32669897 Free PMC article. Review. - MMP13 inhibition rescues cognitive decline in Alzheimer transgenic mice via BACE1 regulation.
Zhu BL, Long Y, Luo W, Yan Z, Lai YJ, Zhao LG, Zhou WH, Wang YJ, Shen LL, Liu L, Deng XJ, Wang XF, Sun F, Chen GJ. Zhu BL, et al. Brain. 2019 Jan 1;142(1):176-192. doi: 10.1093/brain/awy305. Brain. 2019. PMID: 30596903 Free PMC article.
References
- Asami-Odaka A, Ishibashi Y, Kikuchi T, Kitada C, Suzuki N. Long amyloid β-protein secreted from wild-type human neuroblastoma IMR-32 cells. Biochemistry. 1995;34:10272–10278. - PubMed
- Chen Q, Nakajima A, Choi SH, Xiong X, Tang YP. Loss of presenilin function causes Alzheimer's disease-like neurodegeneration in the mouse. J Neurosci Res. 2008;86:1615–1625. - PubMed
- Cleary JP, Walsh DM, Hofmeister JJ, Shankar GM, Kuskowski MA, Selkoe DJ, Ashe KH. Natural oligomers of the amyloid-β protein specifically disrupt cognitive function. Nat Neurosci. 2005;8:79–84. - PubMed
- Cole SL, Vassar R. BACE1 structure and function in health and Alzheimer's disease. Curr Alzheimer Res. 2008;5:100–120. - PubMed
- Doedens JR, Mahimkar RM, Black RA. TACE/ADAM-17 enzymatic activity is increased in response to cellular stimulation. Biochem Biophys Res Commun. 2003;308:331–338. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous