Oppositional effects of serotonin receptors 5-HT1a, 2, and 2c in the regulation of adult hippocampal neurogenesis - PubMed (original) (raw)
Oppositional effects of serotonin receptors 5-HT1a, 2, and 2c in the regulation of adult hippocampal neurogenesis
Friederike Klempin et al. Front Mol Neurosci. 2010.
Abstract
Serotonin (5-HT) appears to play a major role in controlling adult hippocampal neurogenesis and thereby it is relevant for theories linking failing adult neurogenesis to the pathogenesis of major depression and the mechanisms of action of antidepressants. Serotonergic drugs lacked acute effects on adult neurogenesis in many studies, which suggested a surprisingly long latency phase. Here we report that the selective serotonin reuptake inhibitor fluoxetine, which has no acute effect on precursor cell proliferation, causes the well-described increase in net neurogenesis upon prolonged treatment partly by promoting the survival and maturation of new postmitotic neurons. We hypothesized that this result is the cumulative effect of several 5-HT-dependent events in the course of adult neurogenesis. Thus, we used specific agonists and antagonists to 5-HT1a, 2, and 2c receptor subtypes to analyze their impact on different developmental stages. We found that 5-HT exerts acute and opposing effects on proliferation and survival or differentiation of precursor cells by activating the diverse receptor subtypes on different stages within the neuronal lineage in vivo. This was confirmed in vitro by demonstrating that 5-HT1a receptors are involved in self-renewal of precursor cells, whereas 5-HT2 receptors effect both proliferation and promote neuronal differentiation. We propose that under acute conditions 5-HT2 effects counteract the positive proliferative effect of 5-HT1a receptor activation. However, prolonged 5-HT2c receptor activation fosters an increase in late-stage progenitor cells and early postmitotic neurons, leading to a net increase in adult neurogenesis. Our data indicate that serotonin does not show effect latency in the adult dentate gyrus. Rather, the delayed response to serotonergic drugs with respect to endpoints downstream of the immediate receptor activity is largely due to the initially antagonistic and un-balanced action of different 5-HT receptors.
Keywords: adult neurogenesis; dentate gyrus; fluoxetine; serotonin receptors; stem cell.
Figures
Figure 1
Experimental design. (A) Time course of fluoxetine treatment for proliferation and survival experiments. Mice were injected either with NaCl (Vehicle Control) or fluoxetine once followed by one single injection of BrdU 24 h later. The survival paradigm is divided into two parts: Mice were injected first with BrdU followed by a daily administration of either NaCl or fluoxetine over 21 days; a third group received one single injection of fluoxetine followed by NaCl (Vehicle) once daily over 20 days. (B) Time course of serotonin receptor agonist and antagonist treatments for proliferation and survival experiments. Mice were injected once with NaCl (Vehicle Control), the antagonist or agonist followed by one single injection of BrdU after 2 h, and they were killed 24 h later. For cell survival animals were injected first with BrdU followed 8 h later by a daily administration of NaCl, the antagonist or agonist over 7 days.
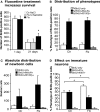
Figure 2
Effects of acute and chronic fluoxetine treatment. (A) Compared to control (Ctr NaCl), chronic but not acute fluoxetine treatment significantly increased the number of BrdU-positive cells in the dentate gyrus. Injection of BrdU 24 h before the beginning of 21 days chronic fluoxetine administration exerted a survival-promoting effect on newborn cells, whereas one single injection of the drug followed by 20 days of saline had no effect. Chronic fluoxetine treatment resulted in a net increase in newborn cells (C) without affecting the proportion of new neurons (BrdU+/NeuN+) (B), but significantly decreased the ratio of BrdU-positive cells expressing the transient postmitotic marker Calretinin (Cr) (D). Here, the increased proportion of BrdU-labeled cells of undetermined phenotype presumably reflects the increase in NeuN-positive cells.
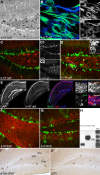
Figure 3
Qualitative results. (A–G) 5-HT1a, 2a and 2c receptor expression pattern in vivo, and in vitro. (A) Anti-5-HT1aR staining (DAB reaction) shows receptor expression in neurons both in the hilus and granule cell layer including the subgranular zone, Scale bar 80 μm. (B) Proliferating precursor cells [Nestin, green **(B1)**] in cell culture show an intense 5-HT1aR expression [blue, **(B2)**], Scale bar 10 μm. (C) 5-HT1a receptor expression in the dentate gyrus [Doublecortin, green (C1); 5-HT1aR, red (C2)]. (D) The receptor is detectable in horizontal type-2 cells revealed as an overlap with DCX-EGFP [green (D1), 5-HT1aR in red (D2). (E) Single focal plane of the hippocampus after in situ hybridization with riboprobe for the 5-HT1a receptor, immunofluorescence for BrdU, and DAPI; the confocal image reveals strong receptor expression in CA1, fainter in the dentate gyrus (DG), and no staining in the CA3 region. (F) 5-HT2a receptor expression pattern (red) in the dentate gyrus reveals a dense staining in the hilus whereas anti-5-HT2c receptor staining [(G), red] mostly marks the granule cell layer (Nestin in green). (H) Expression analysis of 5-HT receptors: PCR analysis was done from reverse transcribed RNA obtained from neural precursor cells under proliferative conditions. There is abundant expression of 5-HT1aR (Lane 1) and 5-HT2aR (Lane 2). Also low levels of 5-HT2c receptors expressed in the precursor cells can be seen (Lane 3). (I,J) Anti-BrdU DAB reaction. A single i.p., injection of the 5-HT1aR agonist 8-OH DPAT 2h pre-BrdU increases the number of BrdU-positive cells in the subgranular zone (SGZ) 24 h later compare to control (CTR in J; GCL, granule cell layer; Scale bar 120 μm).
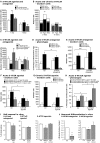
Figure 4
Effects of acute and chronic 5-HT1a, 2, and 2c receptor agonists and antagonists treatment. (A,B) 5-HT1aR effects. (A) Acute treatment with the 5-HT1a receptor agonist 8-OH DPAT produces a significant increase in cell proliferation 1 day after BrdU injection, whereas chronic stimulation for 1 week has no effect. In contrast, acute 5-HT1a receptor blocking with WAY100135 causes no change in cell proliferation but decreases the number of BrdU-positive cells 1 week later. (B) Confocal analysis of the differentiation profile reveals a net increase in the proportion of BrdU/DCX-positive cells after acute stimulation, and a decrease of newborn neurons (BrdU+/NeuN+) upon chronic treatment with the antagonist. (C–H) Effects of acute and chronic 5-HT2 receptor agonist and antagonist treatment, and 5-HT2c receptor stimulation on precursor cell proliferation and differentiation in the adult dentate gyrus. (C) As compared to control, acute treatment with the 5-HT2 receptor antagonist Cinanserin leads to a large increase in the number of proliferating cells 1 day after BrdU injection, but shows no differences 1 week later. In contrast, acute stimulation with the agonist α-methyl-5-HT-maleate as well as chronic treatment over 7 days significantly decreases the number of BrdU-positive cells. Acute 5-HT2c receptor agonist treatment also decreases cell proliferation, but has no effect on survival. Phenotypic analysis reveals a significant decrease in BrdU+/Dcx+ cells after acute 5-HT2R antagonist treatment (D) that result in a largely net increase in the number of BrdU+ cells of undetermined phenotype (E). Phenotypic analysis of acute and chronic effects of the 5-HT2R agonist reveal a net decrease in type-1/2a and type-2b cells after acute treatment (F), and a significantly reduced number of newborn neurons after 7 days (G). Acute 5-HT2c receptor agonist treatment exerts a shift from type-1/2a (which decreases) to type-3 and newly postmitotic cells (which increases), with the type-2b stage being unaffected (H). Data present the absolute number of BrdU-positive cells per dentate gyrus as well as their phenotype by percentage and absolute number, mean ± SD. (I) In vitro, self-renewal potential of neural precursor cells indicated by the capacity to form spheres when plated at clonal densities. Upon 5-HT1aR antagonist (NAN-190) addition as well as upon 5-HT2R stimulation with α-methyl-5-HT the self-renewal potential was significantly decreased, whereas the 5-HT1aR agonist 8-OH DPAT had no effect when added to the culture. (J) Neuronal differentiation of precursor cells was detected by β-III-tubulin antibody staining, and the number of neurons was counted. 5-HT2R antagonist Cinanserin (Cin) potently decreased neuronal differentiation from adult neural precursor cells suggesting an essential role for 5-HT2R in neuronal differentiation. 5-HT2R agonist addition but not 5-HT2cR agonist (WAY161503) also inhibited neuronal differentiation.
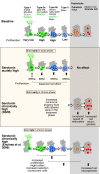
Figure 5
Schematic drawing of effects of Flx treatment and 5-HT receptors stimulation on adult hippocampal neurogenesis. Under baseline levels, from left to right, low proliferative radial glia-like cells (type-1 with GFAP-expression) give rise to three stages of transient amplifying lineage-determined progenitor cells (type-2a, -2b, and 3 with Dcx-expression) to postmitotic immature granule cells (transient Calretinin-expression) and mature neurons. The middle panel highlighted in color, shows the effect of acute and chronically manipulated serotonin levels on proliferation and differentiation. While 5-HT receptors reveal a balanced net effect on earlier stages, the SSRI Flx effect more advanced stages and increases net neurogenesis. The bottom panel in comparison summarizes the data by Encinas et al. (2006), who proposed a selective effect on early precursor cell proliferation and increased net neurogenesis.
Similar articles
- Paradoxical increase in survival of newborn neurons in the dentate gyrus of mice with constitutive depletion of serotonin.
Diaz SL, Narboux-Nême N, Trowbridge S, Scotto-Lomassese S, Kleine Borgmann FB, Jessberger S, Giros B, Maroteaux L, Deneris E, Gaspar P. Diaz SL, et al. Eur J Neurosci. 2013 Sep;38(5):2650-8. doi: 10.1111/ejn.12297. Epub 2013 Jul 10. Eur J Neurosci. 2013. PMID: 23841816 - The Effect of Serotonin-Targeting Antidepressants on Neurogenesis and Neuronal Maturation of the Hippocampus Mediated via 5-HT1A and 5-HT4 Receptors.
Segi-Nishida E. Segi-Nishida E. Front Cell Neurosci. 2017 May 16;11:142. doi: 10.3389/fncel.2017.00142. eCollection 2017. Front Cell Neurosci. 2017. PMID: 28559799 Free PMC article. Review. - Studies on the neuroendocrine role of serotonin.
Jørgensen HS. Jørgensen HS. Dan Med Bull. 2007 Nov;54(4):266-88. Dan Med Bull. 2007. PMID: 18208678 Review. - Proneurogenic Effects of Trazodone in Murine and Human Neural Progenitor Cells.
Bortolotto V, Mancini F, Mangano G, Salem R, Xia E, Del Grosso E, Bianchi M, Canonico PL, Polenzani L, Grilli M. Bortolotto V, et al. ACS Chem Neurosci. 2017 Sep 20;8(9):2027-2038. doi: 10.1021/acschemneuro.7b00175. Epub 2017 Jul 3. ACS Chem Neurosci. 2017. PMID: 28636360
Cited by
- Differential Hippocampal Expression of BDNF Isoforms and Their Receptors Under Diverse Configurations of the Serotonergic System in a Mice Model of Increased Neuronal Survival.
Foltran RB, Stefani KM, Bonafina A, Resasco A, Diaz SL. Foltran RB, et al. Front Cell Neurosci. 2019 Aug 21;13:384. doi: 10.3389/fncel.2019.00384. eCollection 2019. Front Cell Neurosci. 2019. PMID: 31555094 Free PMC article. - Dentate nNOS accounts for stress-induced 5-HT1A receptor deficiency: Implication in anxiety behaviors.
Zhu LJ, Xu C, Ren J, Chang L, Zhu XH, Sun N, Meng GL, Liu MY, Zhang J, Li YY, Tang YL, Zhou QG. Zhu LJ, et al. CNS Neurosci Ther. 2020 Apr;26(4):453-464. doi: 10.1111/cns.13269. Epub 2019 Dec 21. CNS Neurosci Ther. 2020. PMID: 31863649 Free PMC article. - Serotonin is required for exercise-induced adult hippocampal neurogenesis.
Klempin F, Beis D, Mosienko V, Kempermann G, Bader M, Alenina N. Klempin F, et al. J Neurosci. 2013 May 8;33(19):8270-5. doi: 10.1523/JNEUROSCI.5855-12.2013. J Neurosci. 2013. PMID: 23658167 Free PMC article. - Is Adult Hippocampal Neurogenesis Really Relevant for the Treatment of Psychiatric Disorders?
Carli M, Aringhieri S, Kolachalam S, Longoni B, Grenno G, Rossi M, Gemignani A, Fornai F, Maggio R, Scarselli M. Carli M, et al. Curr Neuropharmacol. 2021;19(10):1640-1660. doi: 10.2174/1570159X18666200818194948. Curr Neuropharmacol. 2021. PMID: 32811415 Free PMC article. Review. - Neuroanatomical, Biochemical, and Functional Modifications in Brain Induced by Treatment with Antidepressants.
Khushboo, Siddiqi NJ, de Lourdes Pereira M, Sharma B. Khushboo, et al. Mol Neurobiol. 2022 Jun;59(6):3564-3584. doi: 10.1007/s12035-022-02780-z. Epub 2022 Mar 29. Mol Neurobiol. 2022. PMID: 35352302 Review.
References
- Banasr M., Hery M., Printemps R., Daszuta A. (2004). Serotonin-induced increases in adult cell proliferation and neurogenesis are mediated through different and common 5-HT receptor subtypes in the dentate gyrus and the subventricular zone. Neuropsychopharmacology 29, 450–46010.1038/sj.npp.1300320 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases