Glycan analysis and influenza A virus infection of primary swine respiratory epithelial cells: the importance of NeuAc{alpha}2-6 glycans - PubMed (original) (raw)
Glycan analysis and influenza A virus infection of primary swine respiratory epithelial cells: the importance of NeuAc{alpha}2-6 glycans
Allen C Bateman et al. J Biol Chem. 2010.
Abstract
To better understand influenza virus infection of pigs, we examined primary swine respiratory epithelial cells (SRECs, the primary target cells of influenza viruses in vivo), as a model system. Glycomic profiling of SRECs by mass spectrometry revealed a diverse range of glycans terminating in sialic acid or GalαGal. In terms of sialylation, α2-6 linkage was more abundant than α2-3, and NeuAc was more abundant than NeuGc. Virus binding and infection experiments were conducted to determine functionally important glycans for influenza virus infection, with a focus on recently emerged swine viruses. Infection of SRECs with swine and human viruses resulted in different infectivity levels. Glycan microarray analysis with a high infectivity "triple reassortant" virus ((A/Swine/MN/593/99 (H3N2)) that spread widely throughout the North American swine population and a lower infectivity human virus isolated from a single pig (A/Swine/ONT/00130/97 (H3N2)) showed that both viruses bound exclusively to glycans containing NeuAcα2-6, with strong binding to sialylated polylactosamine and sialylated N-glycans. Treatment with mannosamine precursors of sialic acid (to alter NeuAc/NeuGc abundances) and linkage-specific sialidases prior to infection indicated that the influenza viruses tested preferentially utilize NeuAcα2-6-sialylated glycans to infect SRECs. Our data indicate that NeuAcα2-6-terminated polylactosamine and sialylated N-glycans are important determinants for influenza viruses to infect SRECs. As NeuAcα2-6 polylactosamine glycans play major roles in human virus infection, the importance of these receptor components in virus infection of swine cells has implications for transmission of viruses between humans and pigs and for pigs as possible adaptation hosts of novel human influenza viruses.
Figures
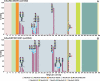
FIGURE 1.
MALDI-TOF MS profiles of the permethylated _N_-linked glycans derived from SRECs. For complete annotation of the spectrum, see
supplemental Table S1
. Data were obtained from the 50% acetonitrile fraction, and all molecular ions are present in sodiated form ([M + Na]+).
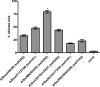
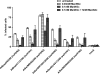
FIGURE 2.
Flow cytometry-based quantification of virus infectivity levels in SRECs. The data are mean ± S.E. of three independent experiments performed in triplicate. *, p < 0.01 compared with infectivity level of each of the other viruses.
FIGURE 3.
Glycan microarray analysis of Sw/MN and Sw/ONT viruses. Sw/MN (A) and Sw/ONT (B) binding to glycans was performed on microarray version 4.1 from the Consortium for Functional Glycomics. Results shown are the average of four replicate spots ± S.E. after the highest and lowest readings of six were excluded, with the highest value set to 100. As the binding of all asialo- and α2–3-sialylated glycans was below 1.5%, the structures of only five α2–3-sialylated glycans are plotted on the graph for clarity of presentation. For complete glycan sequences and relative luciferase units of viruses binding to all glycans see
supplemental Table S4
.
FIGURE 4.
ManNGc and ManNAc treatment of SRECs followed by virus infection. SRECs were grown in the presence of ManNGc and/or ManNAc for 2 days prior to infection. Precursor molecules were solubilized in DMSO and added to a final concentration of 0.05% DMSO. A DMSO control showed no change in infectivity (data not shown). The data shown are the means ± S.E. of three independent experiments performed in triplicate. *, p < 0.01 compared with untreated cells.
FIGURE 5.
Partial MALDI-TOF MS profiles of the permethylated _N_-linked glycans derived from SRECs after digestion with sialidase S or sialidase A. Data were obtained from the 50% acetonitrile fraction and all molecular ions are present in sodiated form ([M + Na]+). Sialylated species are annotated in red (see
supplemental Table S1
).
FIGURE 6.
Sialidase treatment of SRECs prior to virus infection. The data shown are the mean ± S.E. of three independent experiments performed in triplicate. *, p < 0.01 compared with untreated cells.
Similar articles
- Human H3N2 Influenza Viruses Isolated from 1968 To 2012 Show Varying Preference for Receptor Substructures with No Apparent Consequences for Disease or Spread.
Gulati S, Smith DF, Cummings RD, Couch RB, Griesemer SB, St George K, Webster RG, Air GM. Gulati S, et al. PLoS One. 2013 Jun 21;8(6):e66325. doi: 10.1371/journal.pone.0066325. Print 2013. PLoS One. 2013. PMID: 23805213 Free PMC article. - Infectivity phenotypes of H3N2 influenza A viruses in primary swine respiratory epithelial cells are controlled by sialic acid binding.
Bateman AC, Busch MG, Karasin AI, Olsen CW. Bateman AC, et al. Influenza Other Respir Viruses. 2012 Nov;6(6):424-33. doi: 10.1111/j.1750-2659.2012.00333.x. Epub 2012 Feb 21. Influenza Other Respir Viruses. 2012. PMID: 22353399 Free PMC article. - Identification of amino acids in the HA of H3 influenza viruses that determine infectivity levels in primary swine respiratory epithelial cells.
Busch MG, Bateman AC, Landolt GA, Karasin AI, Brockman-Schneider RA, Gern JE, Suresh M, Olsen CW. Busch MG, et al. Virus Res. 2008 May;133(2):269-79. doi: 10.1016/j.virusres.2008.01.014. Epub 2008 Mar 10. Virus Res. 2008. PMID: 18329747 - Adaptation of influenza viruses to human airway receptors.
Thompson AJ, Paulson JC. Thompson AJ, et al. J Biol Chem. 2021 Jan-Jun;296:100017. doi: 10.1074/jbc.REV120.013309. Epub 2020 Nov 22. J Biol Chem. 2021. PMID: 33144323 Free PMC article. Review. - Glycans as receptors for influenza pathogenesis.
Viswanathan K, Chandrasekaran A, Srinivasan A, Raman R, Sasisekharan V, Sasisekharan R. Viswanathan K, et al. Glycoconj J. 2010 Aug;27(6):561-70. doi: 10.1007/s10719-010-9303-4. Epub 2010 Aug 24. Glycoconj J. 2010. PMID: 20734133 Free PMC article. Review.
Cited by
- Respiratory mucus as a virus-host range determinant.
Wallace LE, Liu M, van Kuppeveld FJM, de Vries E, de Haan CAM. Wallace LE, et al. Trends Microbiol. 2021 Nov;29(11):983-992. doi: 10.1016/j.tim.2021.03.014. Epub 2021 Apr 16. Trends Microbiol. 2021. PMID: 33875348 Free PMC article. Review. - Pandemic Swine H1N1 Influenza Viruses with Almost Undetectable Neuraminidase Activity Are Not Transmitted via Aerosols in Ferrets and Are Inhibited by Human Mucus but Not Swine Mucus.
Zanin M, Marathe B, Wong SS, Yoon SW, Collin E, Oshansky C, Jones J, Hause B, Webby R. Zanin M, et al. J Virol. 2015 Jun;89(11):5935-48. doi: 10.1128/JVI.02537-14. Epub 2015 Mar 25. J Virol. 2015. PMID: 25810540 Free PMC article. - _N_-Glycolylneuraminic Acid Binding of Avian and Equine H7 Influenza A Viruses.
Spruit CM, Zhu X, Tomris I, Ríos-Carrasco M, Han AX, Broszeit F, van der Woude R, Bouwman KM, Luu MMT, Matsuno K, Sakoda Y, Russell CA, Wilson IA, Boons GJ, de Vries RP. Spruit CM, et al. J Virol. 2022 Mar 9;96(5):e0212021. doi: 10.1128/jvi.02120-21. Epub 2022 Jan 19. J Virol. 2022. PMID: 35044215 Free PMC article. - Filter-aided N-glycan separation (FANGS): a convenient sample preparation method for mass spectrometric N-glycan profiling.
Abdul Rahman S, Bergström E, Watson CJ, Wilson KM, Ashford DA, Thomas JR, Ungar D, Thomas-Oates JE. Abdul Rahman S, et al. J Proteome Res. 2014 Mar 7;13(3):1167-76. doi: 10.1021/pr401043r. Epub 2014 Feb 6. J Proteome Res. 2014. PMID: 24450425 Free PMC article. - Comprehensive overview of COVID-19 based on current evidence.
Kang Y, Xu S. Kang Y, et al. Dermatol Ther. 2020 Sep;33(5):e13525. doi: 10.1111/dth.13525. Epub 2020 May 22. Dermatol Ther. 2020. PMID: 32378801 Free PMC article. Review.
References
- Kida H., Ito T., Yasuda J., Shimizu Y., Itakura C., Shortridge K. F., Kawaoka Y., Webster R. G. (1994) J. Gen. Virol. 75, 2183–2188 - PubMed
- Landolt G. A., Olsen C. W. (2007) Anim. Health Res. Rev. 8, 1–21 - PubMed
- Brown I. H. (2000) Vet. Microbiol. 74, 29–46 - PubMed
- Scholtissek C. (1990) Med. Princ. Pract. 2, 65–71
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials