mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists - PubMed (original) (raw)
mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists
Nanxin Li et al. Science. 2010.
Abstract
The rapid antidepressant response after ketamine administration in treatment-resistant depressed patients suggests a possible new approach for treating mood disorders compared to the weeks or months required for standard medications. However, the mechanisms underlying this action of ketamine [a glutamate N-methyl-D-aspartic acid (NMDA) receptor antagonist] have not been identified. We observed that ketamine rapidly activated the mammalian target of rapamycin (mTOR) pathway, leading to increased synaptic signaling proteins and increased number and function of new spine synapses in the prefrontal cortex of rats. Moreover, blockade of mTOR signaling completely blocked ketamine induction of synaptogenesis and behavioral responses in models of depression. Our results demonstrate that these effects of ketamine are opposite to the synaptic deficits that result from exposure to stress and could contribute to the fast antidepressant actions of ketamine.
Figures
Fig. 1
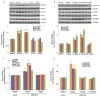
Ketamine transiently and dose-dependently activates mTOR signaling in rat prefrontal cortex (PFC). (A) Time course of ketamine (10 mg/kg, i.p.) induced mTOR signaling determined by Western blot analysis of phospho-mTOR (pmTOR), phospho-4E-BP1 (p4E-BP1), and phospho-p70S6K (pp70S6K) in synaptoneurosomes of PFC. Levels of total mTOR, GAPDH and p70S6K were also determined. (B) Dose-dependent activation, determined 1 hr after ketamine administration, of pmTOR, p4E-BP1 and pp70S6K. (C) Pre-treatment (10 min) with NBQX (10 mg/kg, i.p.) blocked ketamine (10 mg/kg, i.p.) activation of pmTOR, p4E-BP1, and pp70S6K, as well as upstream signaling kinases phospho-ERK (pERK) and phospho-Akt (pAkt) (analyzed 1 hr after ketamine). Levels of pERK1 and pERK2 were similarly regulated and were combined for quantitative analysis. (D) Pre-treatment (30 min) with inhibitors of ERK (U0126, 20 nmol, ICV) or PI-3k/Akt (LY294002, 20 nmol, ICV) abolished ketamine (10 mg/kg, i.p.) activation of mTOR signaling proteins (analyzed 1 hr after ketamine administration). Values represent mean ± SEM [n = 4 animals; * P < 0.05; ** P < 0.01, Analysis of Variance (ANOVA)].
Fig. 2
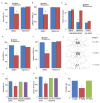
Ketamine rapidly increases synaptic proteins and spine number. (A) Time course for ketamine (10 mg/kg, ip) induction of synaptic proteins ARC, synapsin I, PSD95, and GluR1 in synaptoneurosomes of prefrontal cortex (PFC), and (B) blockade by pre-treatment (30 min) with a selective mTOR inhibitor rapamycin (0.2 nmol, ICV) (values represent mean ± SEM, n = 4 – 6 animals; *P < 0.05; **P < 0.01, ANOVA.) (C) Ketamine increased spine density in medial PFC, analyzed by two-photon microscopy 24 hr after treatment. Representative images are shown of high magnification Z-stack projections of apical tuft segments of Neurobiotin-labeled layer V pyramidal cells (Scale: 5 μm). (D) Results are the mean ± SEM (8 cells from 4 rats in each group; *P < 0.05; **P < 0.01, _t_-test). (E) Ketamine enhanced mPFC layer V pyramidal cell EPSC responses. Sample whole cell voltage-clamp recordings of 5-HT and hypocretin-induced EPSCs in slices (24 hr post-ketamine). (F) Cumulative probability distributions showing significant increases in amplitude (p < 0.0001, KS-z vale = 6.5 for 5-HT and 6.7 for Hcrt) and (H) frequency of 5-HT- and hypocretin-induced EPSCs (n = 12 neurons/group; *P < 0.05, _t_-test). Ketamine-induction of spine density and function were blocked by rapamycin infusions (C–G)
Fig. 3
Rapid behavioral actions of ketamine require mTOR signaling. Rapamycin was infused (0.2 nmol, ICV) 30 min prior to ketamine (10 mg/kg, ip), and responses in the FST (A), NSFT (B), or LH (C) paradigms was determined. Infusion of rapamycin (0.01 nmol) into the medial prefrontal cortex (PFC) blocked the antidepressant actions of ketamine (10 mg/kg, ip) in the FST (D) and NSFT (E). (F) Location of rapamycin infusions in the PFC (infusion sites are the same on the right and left sides due to the use of bilateral cannulae). Pre-treatment with inhibitors of ERK (U0126, 20 nmol, ICV) or PI3 kinase/Akt (LY294002, 20 nmol, ICV) blocked the behavioral effects of ketamine in FST (G) and NSFT (H). (I) Low (10 mg/kg) but not a high anesthetic dose (80 mg/kg) of ketamine produced an antidepressant action in the FST. Values represent mean ± SEM (n = 6–8 animals; *P < 0.05; **P < 0.01, ANOVA).
Fig. 4
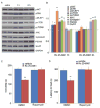
Selective NR2B antagonist Ro 25-6981 activates mTOR signaling and produces rapamycin-sensitive behavioral effects. (A, B) Effects of Ro 25-6981 (10 mg/kg, ip) on pmTOR, p4E-BP1, pp70S6K, pERK, pAkt, Arc, synapsin I, PSD95, and GluR1 in prefrontal cortex. (C–D) Pre-treatment with rapamycin (0.2 nmol, ICV) abolished the actions of Ro 25-6981 in FST (C) and NSFT (D). Values represent mean ± SEM (n = 6–8 animals; *P < 0.05; **P < 0.01, ANOVA).
Comment in
- Neuroscience. A glutamate pathway to faster-acting antidepressants?
Cryan JF, O'Leary OF. Cryan JF, et al. Science. 2010 Aug 20;329(5994):913-4. doi: 10.1126/science.1194313. Science. 2010. PMID: 20724626 No abstract available. - Psychiatric disorders: Ketamine modifies mood through mTOR.
Welberg L. Welberg L. Nat Rev Neurosci. 2010 Oct;11(10):666. doi: 10.1038/nrn2916. Nat Rev Neurosci. 2010. PMID: 21080533 No abstract available. - Role of the mTOR signaling pathway in the rapid antidepressant action of ketamine.
Hashimoto K. Hashimoto K. Expert Rev Neurother. 2011 Jan;11(1):33-6. doi: 10.1586/ern.10.176. Expert Rev Neurother. 2011. PMID: 21158553
Similar articles
- Glutamate N-methyl-D-aspartate receptor antagonists rapidly reverse behavioral and synaptic deficits caused by chronic stress exposure.
Li N, Liu RJ, Dwyer JM, Banasr M, Lee B, Son H, Li XY, Aghajanian G, Duman RS. Li N, et al. Biol Psychiatry. 2011 Apr 15;69(8):754-61. doi: 10.1016/j.biopsych.2010.12.015. Epub 2011 Feb 3. Biol Psychiatry. 2011. PMID: 21292242 Free PMC article. - A neurotrophic hypothesis of depression: role of synaptogenesis in the actions of NMDA receptor antagonists.
Duman RS, Li N. Duman RS, et al. Philos Trans R Soc Lond B Biol Sci. 2012 Sep 5;367(1601):2475-84. doi: 10.1098/rstb.2011.0357. Philos Trans R Soc Lond B Biol Sci. 2012. PMID: 22826346 Free PMC article. Review. - Scopolamine rapidly increases mammalian target of rapamycin complex 1 signaling, synaptogenesis, and antidepressant behavioral responses.
Voleti B, Navarria A, Liu RJ, Banasr M, Li N, Terwilliger R, Sanacora G, Eid T, Aghajanian G, Duman RS. Voleti B, et al. Biol Psychiatry. 2013 Nov 15;74(10):742-9. doi: 10.1016/j.biopsych.2013.04.025. Epub 2013 Jun 14. Biol Psychiatry. 2013. PMID: 23751205 Free PMC article. - Signaling pathways underlying the rapid antidepressant actions of ketamine.
Duman RS, Li N, Liu RJ, Duric V, Aghajanian G. Duman RS, et al. Neuropharmacology. 2012 Jan;62(1):35-41. doi: 10.1016/j.neuropharm.2011.08.044. Epub 2011 Sep 2. Neuropharmacology. 2012. PMID: 21907221 Free PMC article. Review. - Essential roles of neuropeptide VGF regulated TrkB/mTOR/BICC1 signaling and phosphorylation of AMPA receptor subunit GluA1 in the rapid antidepressant-like actions of ketamine in mice.
Shen M, Lv D, Liu X, Li S, Chen Y, Zhang Y, Wang Z, Wang C. Shen M, et al. Brain Res Bull. 2018 Oct;143:58-65. doi: 10.1016/j.brainresbull.2018.10.004. Epub 2018 Oct 11. Brain Res Bull. 2018. PMID: 30316917
Cited by
- Antidepressant-like effect of guanosine involves activation of AMPA receptor and BDNF/TrkB signaling.
Rosa PB, Bettio LEB, Neis VB, Moretti M, Kaufmann FN, Tavares MK, Werle I, Dalsenter Y, Platt N, Rosado AF, Fraga DB, Heinrich IA, Freitas AE, Leal RB, Rodrigues ALS. Rosa PB, et al. Purinergic Signal. 2021 Jun;17(2):285-301. doi: 10.1007/s11302-021-09779-6. Epub 2021 Mar 13. Purinergic Signal. 2021. PMID: 33712981 Free PMC article. - AMPAkines Target the Nucleus Accumbens to Relieve Postoperative Pain.
Su C, Lin HY, Yang R, Xu D, Lee M, Pawlak N, Norcini M, Sideris A, Recio-Pinto E, Huang D, Wang J. Su C, et al. Anesthesiology. 2016 Nov;125(5):1030-1043. doi: 10.1097/ALN.0000000000001336. Anesthesiology. 2016. PMID: 27627816 Free PMC article. - Activation of the p11/SMARCA3/Neurensin-2 pathway in parvalbumin interneurons mediates the response to chronic antidepressants.
Umschweif G, Medrihan L, McCabe KA, Sagi Y, Greengard P. Umschweif G, et al. Mol Psychiatry. 2021 Jul;26(7):3350-3362. doi: 10.1038/s41380-021-01059-4. Epub 2021 Mar 15. Mol Psychiatry. 2021. PMID: 33723417 Free PMC article. - Rapamycin Augments the NMDA-Mediated TNF Suppression of MRSA-Stimulated RAW264.7 Murine Macrophages.
Spentzas T, Shappley RK, Savorgnan F, Meals E, English BK. Spentzas T, et al. Int J Inflam. 2012;2012:542727. doi: 10.1155/2012/542727. Epub 2012 Oct 10. Int J Inflam. 2012. PMID: 23094196 Free PMC article. - Repeated Dosing of Ketamine in the Forced Swim Test: Are Multiple Shots Better Than One?
Weston RG, Fitzgerald PJ, Watson BO. Weston RG, et al. Front Psychiatry. 2021 May 11;12:659052. doi: 10.3389/fpsyt.2021.659052. eCollection 2021. Front Psychiatry. 2021. PMID: 34045982 Free PMC article. Review.
References
- Kessler RC, et al. JAMA. 2003;289:3095. - PubMed
- Trivedi MH, et al. Am J Psychiatry. 2006;163:28. - PubMed
- Berman RM, et al. Biol Psychiatry. 2000;47:351. - PubMed
- Zarate CA, Jr, et al. Arch Gen Psychiatry. 2006;63:856. - PubMed
- Krystal JH. Swiss Med Wkly. 2007;137:215. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P01 MH025642/MH/NIMH NIH HHS/United States
- 2P01 MH25642/MH/NIMH NIH HHS/United States
- P01 MH025642-30/MH/NIMH NIH HHS/United States
- R01 MH045481-13/MH/NIMH NIH HHS/United States
- MH45481/MH/NIMH NIH HHS/United States
- R01 MH045481-14/MH/NIMH NIH HHS/United States
- P01 MH025642-32/MH/NIMH NIH HHS/United States
- R01 MH045481-15/MH/NIMH NIH HHS/United States
- R01 MH045481/MH/NIMH NIH HHS/United States
- P01 MH025642-31/MH/NIMH NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous