TRPM7 regulates quiescent/proliferative metabolic transitions in lymphocytes - PubMed (original) (raw)
TRPM7 regulates quiescent/proliferative metabolic transitions in lymphocytes
Jaya Sahni et al. Cell Cycle. 2010.
Abstract
A unique property of lymphocytes among all body tissues is their capacity for rapid proliferation in the context of responding to infectious challenges. Lymphocyte proliferation involves a transition from a quiescent metabolic state adjusted to maintain cellular energy homeostasis, to a proliferative metabolic state in which aerobic glycolysis is used to generate energy and biosynthetic precursors necessary for the accumulation of cell mass. Here we show that modulation of TRPM7 channel function in tumor B-lymphocytes directly induces quiescent/proliferative metabolic transitions. As TRPM7 is widely expressed outside of the immune system, our results suggest that TRPM7 may play an active role in regulating metabolic transitions associated with rapid cellular proliferation and malignancy.
Figures
Figure 1
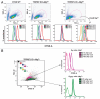
proliferation arrested TRPM7-KO cells exhibit stable CFSE staining upon transition to regular media. (A) TRPM7-deficient cells transitioned to regular media for 24 hours undergo proliferative arrest and show stable CFSE staining. Top part: Forward scatter vs. side scatter analysis displaying cell size distribution of WT cells in regular media, TRPM7-deficient cells in growth-supporting media with supplemental Mg2+ and TRPM7-KO cells transitioned to regular media for 24 hours without and with re-addition of 15 mM Mg2+. All cell lines were labeled with CSFE 24 hrs post transition of TRPM7-KO cells to regular media. Data was acquired on BD LSRII flow cytometer and analyzed by Flowjo (Tree Star, Inc.; Ashland, Oregon). Bottom part: CFSE-labeled cells were acquired at 9, 24 and 32 hours and analyzed by Flowjo. By 25 hours (time not including 24 hours post transition), TRPM7-deficient cells stop undergoing further rounds of proliferation in regular media (-media) without 15 mM supplemental Mg2+ and display substantially stable CFSE staining (3rd bottom part). Provision of supplemental Mg2+ at 24 hrs post transition allowed recovery of only a small proportion of cells by 32 hours (4th bottom part). (B) Proliferation-arrested TRPM7-KO cells resume cell division in presence of supplemental Mg2+. TRPM7-deficient cells cultured in regular media for 24 hours were labeled with CFSE and sorted on the basis of their cell size (smaller G0/G1 and larger G2 cells; see Suppl. Fig. 1) followed by provision of 15 mM supplemental Mg2+ to both populations. Further reacquisition of these two populations at 7, 23 and 49 hours indicated that both G1 and G2 cells are able to resume cell division.
Figure 2
A signature protein of cellular quiescence, p27kip1, is upregulated in proliferation-arrested TRPM7-KO cells. (A) Levels of p27kip1 are significantly elevated in non-proliferating TRPM7-KO cells. Left part: WT, TRPM7-KO cells and TRPM7-KO cells transitioned to regular media were analyzed for p27 expression at 11 and 24 hours post transition. At 11 hours, levels of p27 were elevated in TRPM7-deficient cells transitioned to regular media and were even significantly high at 24 hours post transition compared with WT cells growing in regular media and TRPM7-KO cells in growth-supporting media either with or without rapamycin, a mTO R inhibitor. Right part: Similar to the experiment in the left part, WT, proliferating TRPM7-deficient cells and non-proliferating TRPM7-KO cells were treated with a microtubule inhibitor, nocodazole for 24 hours. Exposure of cells to nocodazole resulted in p27 expression in both WT as well as proliferating TRPM7-KO cells but the levels were considerably lower than those observed in non-proliferating TRPM7-deficient cells. (B) Analysis of the fold change in p27kip1 from three independent experiments is shown. The graph shows averages plus standard errors of the means (error bars) and p values for the fold change were calculated using Student's t test. A 2.7-fold increase in p27 expression was observed by 25 hours in TRPM7-KO cells transitioned to regular media and found to be statistically significant compared to WT cells in regular media at 0 and 11 hours (p = 0.0001 and p = 0.0006) as well as TRPM7-KO cells cultured in growth-supporting media with 15 mM Mg2+ at similar time points (p = 0.0001 and p = 0.001).
Figure 3
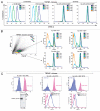
Non-proliferating TRPM7-deficient cells display low RNA content, significantly downregulated store-operated Ca2+ entry (SOCE) and oxygen consumption rate analogous to primary lymphocytes. (A) TRPM7-deficient cells transitioned to regular media exhibit reduced RNA and SOCE. Top left part: Flow cytometric analysis of WT cells in regular media, TRPM7-KO cells in growth-supporting media with/without rapamycin (26 hrs) and TRPM7-KO cells transitioned to regular media for 26 hours stained with DAPI for DNA (Y-axis) and Pyronin Y for RNA (X-axis) content. TRPM7-deficient cells in regular media exhibited a high proportion of Pyronin Y negative cells that are in G0 compared to proliferating WT and TRPM7-KO cells as well as TRPM7-KO treated with rapamycin. The analysis was performed on the same day. Top middle part: TRPM7-KO cells were transitioned to regular media for 24 and 48 hours and their surface IgM expression was analyzed with an anti-IgM antibody directly conjugated to FITC (Bethyl laboratories). WT cells in regular media and TRPM7-KO cells in Mg2+-supplemented media displayed higher surface IgM expression compared to TRPM7-deficient cells transitioned to regular media, consistent with their smaller cell size. Top right part: Store-operated calcium entry was evaluated in WT, TRPM7-KO cells in growth-supporting media with 15 mM Mg2+ and TRPM7-KO cells induced into a non-proliferative state upon transition to regular media for 24 and 52 hours. Cells were labeled with Ca2+-binding dye, indo-1 and acquired on BD LSRII cytometer. Flow kinetic profiles are shown comparing the mean indo-1 ratio (violet/blue) as a function of time before and after stimulation with M4 followed by addition of 1 mM Ca2+. A significant reduction in SOCE was observed in TRPM7-deficient cells cultured in regular media for 24 and 52 hours. Lower left part: WT cells growing in regular media, TRPM7-KO cells in Mg2+-supplemented media and proliferation-arrested TRPM7-deficient cells (24 hours) were analyzed for SOCE upon treatment with thapsigargin. Flow kinetic profiles obtained were similar to what was observed with M4 stimulation (top right part). Lower right part: Changes in intracellular calcium over time for TRPM7-KO cells in media with supplemental Mg2+ and in regular media without 15 mM Mg2+. The response from the whole population of cells from one representative experiment is shown. Upon addition of 1 mM Ca2+, the ion flux in the proliferation-arrested TRPM7-deficient cells (right dot plot) is significantly reduced as compared to the cells in growth-supporting media (left dot plot). Live/dead cell differentiation was done by propidium iodide (PI) staining in all experiments. (B) Proliferation-arrested TRPM7-deficient cells lack Crabtree effect despite functional mitochondria. Left part: A flow cell approach was used for measuring the oxygen consumption rate (OCR) in proliferation-arrested TRPM7-deficient cells compared to WT DT40 cells cultured in regular media and TRPM7-deficient cells in growth-supporting media. Non-proliferating TRPM7-KO cells showed a considerable drop in the OCR, closely resembling the oxygen consumption rate of quiescent primary lymphocytes, when compared to WT and TRPM7-KO cells in Mg2+-supplemented media. Right part: Mitochondrial function was evaluated by determination of mitochondrial membrane potential in WT, TRPM7-deficient cells in growth-supporting media and non-proliferating TRPM7-KO cells transitioned to regular media for 24 hours. While cells labeled with MitoProbe JC-1 displayed mitochondria that retained their normal membrane potential, treatment with CCCP for 5 minutes led to a complete disruption of their mitochondrial electrochemical gradient, which was observed as the shift of JC-1 to its monomeric form (FL-1).
Figure 4
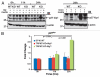
Induction of TRPM7 by doxycycline or provision of supplemental Mg2+ allows proliferation-arrested TRPM7-deficient cells to re-enter the cell cycle. (A) TRPM7-Δ-kinase cells transitioned to regular media display proliferation arrest in both G0/G1 and G2 populations. TRPM7-Δ-kinase cells cultured in growth-supporting media were transitioned to regular media for 24 hours following which they were sorted on the basis of their size scatter (as shown in B) into G0/G1 and G2 populations, commensurate to TRPM7-KO cells. Cells were labeled with CFSE along with positive controls-proliferating TRPM7-Δ-kinase cells in growth supporting media and TRPM7-Δ-kinase cells induced with doxycycline. Comparative analysis showed that while the positive controls proliferated and led to subsequent partitioning of the dye, proliferation arrested and sorted cells in G0/G1 and G2 transitioned to regular media, displayed stable CFSE staining and did not undergo any further cell divisions. (B) Proliferation-arrested TRPM7-Δ-kinase cells are able to recommence proliferation upon doxycycline induction or provision of 15 mM Mg2+. CFSE-labeled sorted G0/G1 and G2 populations were either induced with doxycycline (dox; 1 µg/ml) for TRPM7-Δ-kinase expression or replenished with supplemental Mg2+. By 72 hours, a sizable magnitude of cells were able to exit cell cycle arrest in both G0/G1 as well as G2 populations and resume cell division. However, TRPM7-Δ-kinase cells provided with doxycycline exhibited a lag in resumption of cell division as compared to the cells provided with supplemental Mg2+, which could be attributed to the time required by the dox-induced cells to initiate protein synthesis. (C) TRPM7-Δ-kinase expression drives both G0/G1 and G2 proliferation-arrested cells to reenter cell cycle. TRPM7-Δ-kinase cells transitioned to regular media for 24 hours were sorted into G0/G1 and G2 populations and labeled with CFSE. Left part: Post-sort, cells were transitioned to growth-supporting media or induced with doxycycline. Non-proliferating, stable CFSE labeled cells and proliferating, CFSE-low populations from doxycycline-induced G2 cells were further sorted at 64 hours, lysed and immunoprecipitated with anti-HA antibody. The immunoprecipitates were run on 8% SDS-PAGE and immunoblotted with anti-HA antibody for expression analysis of HA-tagged TRPM7-Δ-kinase. Right part: Similar CFSE-labeled populations, as mentioned in the left part, were sorted at 87 hours for doxycycline induced G0/G1 cells (stable CFSE labeled and CFSE low populations) expressing TRPM7-Δ-kinase and uninduced cells provided with 15 mM supplemental Mg2+. Cells were fixed, permeabilized and labeled with anti-HA/anti-mouse PE antibodies for detection of TRPM7-Δ-kinase by flow cytometry on BD LSRII. A dramatic increase in fluorescence was observed in proliferating CFSE-low, doxycycline-induced G0/G1 population as compared to the non-proliferating, stable CFSE-labeled cells. A small shift in fluorescence was also observed in the uninduced CFSE-low G0/G1 cells, which could be attributed to a leaky promoter.
Comment in
- The primary role of intracellular free Mg2+ in regulating cell growth.
Rubin H. Rubin H. Cell Cycle. 2010 Sep 1;9(17):3396-7. doi: 10.4161/cc.9.17.13050. Cell Cycle. 2010. PMID: 20861664 No abstract available. - TRPM7 and magnesium, metabolism, mitosis: An old path with new pebbles.
Wolf FI, Trapani V. Wolf FI, et al. Cell Cycle. 2010 Sep 1;9(17):3399. doi: 10.4161/cc.9.17.13072. Cell Cycle. 2010. PMID: 20861666 No abstract available.
Similar articles
- TRPM7 and magnesium, metabolism, mitosis: An old path with new pebbles.
Wolf FI, Trapani V. Wolf FI, et al. Cell Cycle. 2010 Sep 1;9(17):3399. doi: 10.4161/cc.9.17.13072. Cell Cycle. 2010. PMID: 20861666 No abstract available. - The primary role of intracellular free Mg2+ in regulating cell growth.
Rubin H. Rubin H. Cell Cycle. 2010 Sep 1;9(17):3396-7. doi: 10.4161/cc.9.17.13050. Cell Cycle. 2010. PMID: 20861664 No abstract available. - TRPM7 is involved in angiotensin II induced cardiac fibrosis development by mediating calcium and magnesium influx.
Yu Y, Chen S, Xiao C, Jia Y, Guo J, Jiang J, Liu P. Yu Y, et al. Cell Calcium. 2014 May;55(5):252-60. doi: 10.1016/j.ceca.2014.02.019. Epub 2014 Mar 11. Cell Calcium. 2014. PMID: 24680379 - TRPM7: a unique channel involved in magnesium homeostasis.
Paravicini TM, Chubanov V, Gudermann T. Paravicini TM, et al. Int J Biochem Cell Biol. 2012 Aug;44(8):1381-4. doi: 10.1016/j.biocel.2012.05.010. Epub 2012 May 24. Int J Biochem Cell Biol. 2012. PMID: 22634382 Review. - TRPM7 and its role in neurodegenerative diseases.
Sun Y, Sukumaran P, Schaar A, Singh BB. Sun Y, et al. Channels (Austin). 2015;9(5):253-61. doi: 10.1080/19336950.2015.1075675. Epub 2015 Jul 28. Channels (Austin). 2015. PMID: 26218331 Free PMC article. Review.
Cited by
- Identification of a Mg2+-sensitive ORF in the 5'-leader of TRPM7 magnesium channel mRNA.
Nikonorova IA, Kornakov NV, Dmitriev SE, Vassilenko KS, Ryazanov AG. Nikonorova IA, et al. Nucleic Acids Res. 2014 Nov 10;42(20):12779-88. doi: 10.1093/nar/gku951. Epub 2014 Oct 17. Nucleic Acids Res. 2014. PMID: 25326319 Free PMC article. - Phosphatidylinositol 4,5-bisphosphate (PIP(2)) controls magnesium gatekeeper TRPM6 activity.
Xie J, Sun B, Du J, Yang W, Chen HC, Overton JD, Runnels LW, Yue L. Xie J, et al. Sci Rep. 2011;1:146. doi: 10.1038/srep00146. Epub 2011 Nov 9. Sci Rep. 2011. PMID: 22180838 Free PMC article. - The Channel-Kinase TRPM7 as Novel Regulator of Immune System Homeostasis.
Nadolni W, Zierler S. Nadolni W, et al. Cells. 2018 Aug 17;7(8):109. doi: 10.3390/cells7080109. Cells. 2018. PMID: 30126133 Free PMC article. Review. - Essential role of Mg2+ in mouse preimplantation embryo development revealed by TRPM7 chanzyme-deficient gametes.
Gupta N, Soriano-Úbeda C, Stein P, Savy V, Papas BN, Ardestani G, Carvacho I, Alfandari D, Williams CJ, Fissore RA. Gupta N, et al. Cell Rep. 2023 Oct 31;42(10):113232. doi: 10.1016/j.celrep.2023.113232. Epub 2023 Oct 11. Cell Rep. 2023. PMID: 37824328 Free PMC article. - A key role for Mg(2+) in TRPM7's control of ROS levels during cell stress.
Chen HC, Su LT, González-Pagán O, Overton JD, Runnels LW. Chen HC, et al. Biochem J. 2012 Aug 1;445(3):441-8. doi: 10.1042/BJ20120248. Biochem J. 2012. PMID: 22587440 Free PMC article.
References
- Schmitz C, Perraud AL, Johnson CO, Inabe K, Smith MK, Penner R, et al. Regulation of vertebrate cellular Mg2+ homeostasis by TRPM7. Cell. 2003;114:191–200. - PubMed
- Yusuf I, Fruman DA. Regulation of quiescence in lymphocytes. Trends Immunol. 2003;24:380–386. - PubMed
- Giaretti W, Abmayr W, Dormer P, Santi L. The G0 in equilibrium G1 transitions of human lymphocytes as monitored by quantitative 14C-uridine autoradiography and high-resolution image analysis. Cytometry. 1985;6:219–225. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous