SEF/IL-17R (SEFIR) is not enough: an extended SEFIR domain is required for il-17RA-mediated signal transduction - PubMed (original) (raw)
SEF/IL-17R (SEFIR) is not enough: an extended SEFIR domain is required for il-17RA-mediated signal transduction
Reiko M Onishi et al. J Biol Chem. 2010.
Abstract
IL-17, the hallmark cytokine of the Th17 population, mediates immunity to extracellular pathogens and promotes autoimmune immunopathology. The signaling mechanisms triggered by the IL-17 receptor (IL-17RA) and related receptors are strikingly different from other cytokine subclasses. Namely, IL-17Rs contain a conserved SEF/IL-17R (SEFIR) subdomain that engages Act1, leading to activation of TRAF6, NF-κB, and other events. Although the SEFIR is critical for signaling, the molecular details of the functional subdomains within IL-17RA remain poorly characterized. Here, we provide a detailed structure-function analysis delineating the C-terminal boundary of the SEFIR-containing region of IL-17RA. We show that functionality of this domain requires a large extension to the previously identified SEFIR motif. In contrast to the SEFIR, this extension is not conserved among IL-17R family members. Surprisingly, Act1 recruitment is not sufficient for downstream signaling activation, whereas ubiquitination of TRAF6 correlates tightly with functional receptors. We further demonstrate that IL-17RA exhibits signaling properties that are nonredundant with other IL-17R family members. Finally, we report that IL-17 signals synergistically with lymphotoxin-α3, using the same signaling motifs within IL-17RA. These studies provide new insight into the structure-function relationships of IL-17RA and reveal distinct signaling differences among IL-17R family members.
Figures
FIGURE 1.
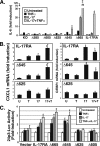
IL-17RA deletion series to evaluate IL-17RA structure-function relationships. A, schematic diagram of the IL-17RA truncation mutants used in this study. ECD, extracellular domain; TM, transmembrane domain. SEFIR and TILL domains are indicated. B, expression of mutant IL-17 receptors. Stable cell lines expressing each receptor were created in the IL-17RA−/− background, and staining for IL-17RA was performed with anti-IL-17RA Abs followed by anti-IgG-phycoerythrin.
FIGURE 2.
The C-terminal boundary of the IL-17RA SEFIR-containing signaling domain lies between amino acid residues 645 and 625. A, induction of IL-6 by IL-17RA deletion mutants. IL-17RA−/− cells stably reconstituted with the indicated IL-17RA mutant series were stimulated with IL-17 (200 ng/ml) and/or suboptimal doses of TNFα (2 ng/ml) for 24 h, and IL-6 secretion was assessed by ELISA of conditioned supernatants, in triplicate. Data are normalized to the untreated sample. *, p < 0.05 compared with untreated samples; ‡, p < 0.05 compared with TNF-treated samples. B, induction of IL-17-dependent gene expression by IL-17RA deletion mutants. IL-17RA−/− cells stably reconstituted with the indicated IL-17RA mutants were stimulated with IL-17 (200 ng/ml) and/or suboptimal doses of TNFα (2 ng/ml) for 8 h, and expression of CXCL1 and C/EBPδ was evaluated by real-time RT-PCR, normalized to an internal GAPDH control. -Fold induction over the untreated samples is shown. *, p < 0.05 compared with untreated samples; ‡, p < 0.05 compared with TNF-treated samples. C, induction of the 24p3 promoter by IL-17RA deletion mutants. IL-17RA−/− cells were transiently transfected with the 24p3-luciferase reporter construct (21) and stimulated with IL-17 and/or TNFα as in A, and lysates were analyzed for luciferase activity, normalized to an internal _Renilla_-luc control. Data are expressed as -fold induction over untreated samples. *, p < 0.05 compared with untreated samples; ‡, p < 0.05 compared with TNF-treated samples. Error bars, S.D.
FIGURE 3.
Activation of TRAF6 but not Act1 requires an intact SEFEX domain. A, association of IL-17RA deletion mutants with Act1. HEK293T cells were transiently transfected with the indicated IL-17RA deletion mutants and Myc-tagged Act1, and lysates were immunoprecipitated with Abs to IL-17RA. IP samples from untreated cells (lanes 1–4) or cells treated with IL-17 for 10 min (lanes 5–8) were separated by SDS-PAGE and immunoblotted (WB) with Abs to Myc (top) or IL-17RA (bottom). The arrows indicate two isoforms of Act1, one of which we have shown to be phosphorylated (23). Note that all lanes were derived from the same gel. Data are representative of at least three experiments. B, Act1 associates preferentially with IL-17RA. HEK293T cells were transiently transfected with HA-tagged receptors, as indicated. Lysates were subjected to IP with anti-HA Abs and immunoblotted with Abs to Myc. C, ubiquitination of TRAF6 by IL-17RA deletion mutants. HEK293T cells were transiently transfected with the indicated IL-17RA deletion mutants and TRAF6, cells were stimulated with or without IL-17 for 15 min, and lysates were immunoprecipitated with Abs to IL-17RA. IP samples were separated by SDS-PAGE and immunoblotted with Abs to TRAF6 (top) or IL-17RA (bottom). Larger ubiquitinated TRAF6 isoforms are indicated. Data are representative of at least three experiments.
FIGURE 4.
Distinct and unique functions of the IL-17RA SEFIR domain. A, alignment of the extended SEFIR domain of IL-17RA family members. ClustalW2 alignment (42) of murine IL-17RA through IL-17RE cytoplasmic tails is depicted. The TILL domain is highlighted in red, and the crucial Val553 residue (required for signal transduction by IL-17RA) (17) is underlined. B, IL-17RA/RC chimeric receptor expression. IL-17RA−/− cells stably transfected with the IL-17RA/RC chimera were stained with anti-IL-17RA Abs and analyzed by flow cytometry. C, replacing the IL-17RA cytoplasmic tail with that of IL-17RC fails to rescue IL-17 induction of IL-6. The IL-17RA/RC-expressing cell line shown in B was treated with IL-17A or IL-17F (200 ng/ml) and/or TNFα (2 ng/ml) for 24 h, and IL-6 in the supernatant was evaluated by ELISA in triplicate. Very similar results were obtained with two other independently derived cell lines (not shown). D, replacing the IL-17RA cytoplasmic tail with that of other IL-17 receptor family members fails to rescue IL-17-induction of the 24p3 promoter. IL-17RA−/− cells were transiently transfected with IL-17RA or the indicated chimeric receptors together with the 24p3-luciferase reporter, and luciferase activity was assessed in triplicate. E, Act1 associates with multiple IL-17R cytoplasmic tails. HEK293T cells were transfected with IL-17RAΔ665 or the chimeric receptors together with Myc-Act1. Lysates were immunoprecipitated with anti-IL-17RA Abs. Lysates (lanes 1–5) and immunoprecipitates (lanes 6–10) were immunoblotted with Abs to Myc (top) or IL-17RA (bottom). Data are representative of at least two experiments. F, ubiquitination of TRAF6 is unique to IL-17RA. HEK293T cells were transiently transfected with the indicated receptors and TRAF6, cells were treated with or without IL-17 for 15 min, and lysates were immunoprecipitated with Abs to IL-17RA. IP samples were separated by SDS-PAGE and immunoblotted (WB) with Abs to TRAF6 (top) or IL-17RA (bottom). Error bars, S.E.
FIGURE 5.
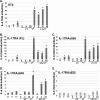
IL-17 synergizes with LTα3, which requires the extended SEFIR domain. A, IL-17 and LTα synergize in a stromal cell line. ST2 stromal cells were treated with IL-17 (200 ng/ml), TNFα (2 ng/ml), and/or LTα3 (2, 4, or 10 ng/ml, as indicated) for 24 h, and supernatants were evaluated for IL-6 in triplicate by ELISA. *, p < 0.05 compared with untreated samples; ‡, p < 0.05 compared with TNF-treated samples. B–E, synergy of IL-17 and LTα3 requires residues in the IL-17RA cytoplasmic tail through amino acid 645. IL-17RA−/− fibroblasts reconstituted with IL-17RA (full-length) or the indicated IL-17RA deletion mutants were treated for 8 or 24 h (not shown) with IL-17, TNFα, and LTα3, and supernatants were evaluated for IL-6 in triplicate as in A.
Similar articles
- Signaling of interleukin-17 family cytokines in immunity and inflammation.
Chang SH, Dong C. Chang SH, et al. Cell Signal. 2011 Jul;23(7):1069-75. doi: 10.1016/j.cellsig.2010.11.022. Epub 2010 Dec 2. Cell Signal. 2011. PMID: 21130872 Free PMC article. Review. - Structure of the unique SEFIR domain from human interleukin 17 receptor A reveals a composite ligand-binding site containing a conserved α-helix for Act1 binding and IL-17 signaling.
Zhang B, Liu C, Qian W, Han Y, Li X, Deng J. Zhang B, et al. Acta Crystallogr D Biol Crystallogr. 2014 May;70(Pt 5):1476-83. doi: 10.1107/S1399004714005227. Epub 2014 Apr 30. Acta Crystallogr D Biol Crystallogr. 2014. PMID: 24816115 Free PMC article. - A CC' loop decoy peptide blocks the interaction between Act1 and IL-17RA to attenuate IL-17- and IL-25-induced inflammation.
Liu C, Swaidani S, Qian W, Kang Z, Sun P, Han Y, Wang C, Gulen MF, Yin W, Zhang C, Fox PL, Aronica M, Hamilton TA, Misra S, Deng J, Li X. Liu C, et al. Sci Signal. 2011 Nov 1;4(197):ra72. doi: 10.1126/scisignal.2001843. Sci Signal. 2011. PMID: 22045852 Free PMC article. - Crystal structure of IL-17 receptor B SEFIR domain.
Zhang B, Liu C, Qian W, Han Y, Li X, Deng J. Zhang B, et al. J Immunol. 2013 Mar 1;190(5):2320-6. doi: 10.4049/jimmunol.1202922. Epub 2013 Jan 25. J Immunol. 2013. PMID: 23355738 Free PMC article. - Act1 modulates autoimmunity through its dual functions in CD40L/BAFF and IL-17 signaling.
Li X. Li X. Cytokine. 2008 Feb;41(2):105-13. doi: 10.1016/j.cyto.2007.09.015. Epub 2007 Dec 3. Cytokine. 2008. PMID: 18061473 Review.
Cited by
- Analysis of cytokine risk factors in the early death of patients with secondary phagocytic lymphocytic histiocytosis.
Li Z, Liu J, Man Y, Liu F, Gao L, Hu P, Zhao R, Wang Y, Yang T. Li Z, et al. Am J Transl Res. 2021 Apr 15;13(4):2388-2398. eCollection 2021. Am J Transl Res. 2021. PMID: 34017397 Free PMC article. - IL-17 induces macrophages to M2-like phenotype via NF-κB.
Shen J, Sun X, Pan B, Cao S, Cao J, Che D, Liu F, Zhang S, Yu Y. Shen J, et al. Cancer Manag Res. 2018 Oct 4;10:4217-4228. doi: 10.2147/CMAR.S174899. eCollection 2018. Cancer Manag Res. 2018. PMID: 30323677 Free PMC article. - Signaling of interleukin-17 family cytokines in immunity and inflammation.
Chang SH, Dong C. Chang SH, et al. Cell Signal. 2011 Jul;23(7):1069-75. doi: 10.1016/j.cellsig.2010.11.022. Epub 2010 Dec 2. Cell Signal. 2011. PMID: 21130872 Free PMC article. Review. - Two TIR-like domain containing proteins in a newly emerging zoonotic Staphylococcus aureus strain sequence type 398 are potential virulence factors by impacting on the host innate immune response.
Patterson NJ, Günther J, Gibson AJ, Offord V, Coffey TJ, Splitter G, Monk I, Seyfert HM, Werling D. Patterson NJ, et al. Front Microbiol. 2014 Dec 9;5:662. doi: 10.3389/fmicb.2014.00662. eCollection 2014. Front Microbiol. 2014. PMID: 25538689 Free PMC article. - IL-17 Signaling: The Yin and the Yang.
Amatya N, Garg AV, Gaffen SL. Amatya N, et al. Trends Immunol. 2017 May;38(5):310-322. doi: 10.1016/j.it.2017.01.006. Epub 2017 Feb 20. Trends Immunol. 2017. PMID: 28254169 Free PMC article. Review.
References
- McGeachy M. J., Cua D. J. (2008) Immunity 28, 445–453 - PubMed
- Korn T., Bettelli E., Oukka M., Kuchroo V. K. (2009) Annu. Rev. Immunol. 27, 485–517 - PubMed
- Shen F., Hu Z., Goswami J., Gaffen S. L. (2006) J. Biol. Chem. 281, 24138–24148 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources