Defining the role of APC in the mitotic spindle checkpoint in vivo: APC-deficient cells are resistant to Taxol - PubMed (original) (raw)
Defining the role of APC in the mitotic spindle checkpoint in vivo: APC-deficient cells are resistant to Taxol
S Radulescu et al. Oncogene. 2010.
Abstract
Mutations in the adenomatous polyposis coli (APC) tumour suppressor are the key initiating event of colorectal cancer. Although the control of WNT signalling is well established as a central tumour-suppressive function, the significance of APC in regulating chromosome instability is less well established. In this study, we test whether APC-deficient cells have a functional spindle assembly checkpoint (SAC) in vivo by examining the response of these cells to Taxol and Vinorelbine. We also show for the first time that APC deficiency compromises the arrest response to Taxol in vivo. This effect is independent of the role that APC has in WNT signalling. At higher levels of Taxol, APC-deficient cells arrest as efficiently as wild-type cells. Importantly, this dose of Taxol strongly suppresses intestinal tumourigenesis in models of benign (APC(Min/+) mouse) and invasive (AhCreER(+)APC(fl/+)PTEN(fl/fl)) cancer. In contrast to intestinal enterocytes with a general SAC defect because of Bub1 (budding uninhibited by benzimidazole 1) deletion, APC-deficient enterocytes arrest equivalently to wild type when treated with Vinorelbine. This suggests that the failed arrest in response to Taxol is because of a specific defect in microtubule stabilization following Taxol treatment rather than a general role of the APC protein in the mitotic spindle checkpoint. In summary, this study clarifies the role of APC as a mitotic spindle checkpoint protein in vivo and shows that APC-deficient cells have a compromised response to Taxol.
Figures
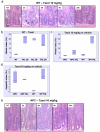
Figure 1. APC-deficient intestinal cells are resistant to low level Taxol treatment
Mice injected with either vehicle (NT), 10, 20 or 30mg/kg Taxol IP (n≥3) were sacrificed 6h post-injection and the levels of mitotic arrest and apoptosis in intestinal crypts were scored using H+E staining. Mitotic and apoptotic index was expressed as percentage of cells per crypt. As intestinal enterocytes enter G2 phase they move out of the crypt lining and into the lumen where they will undergo mitosis and cytokinesis before retuning back to the single cell layer crypt lining. However enterocytes can undergo apoptosis anywhere in the crypt. (a) Representative H+E staining of crypts from WT mice treated with Taxol 10mg/kg and sacrificed at 3h, 6h, 9h or 24h post-injection. Black arrows – mitotic figure, red arrow – apoptotic figure. Scale bar 20μm. (b) Mitotic index in WT intestinal crypts from mice treated with vehicle versus increasing concentrations of Taxol, p<0.026. (c) and (d) Mitotic and apoptotic index in intestinal crypts from vehicle or Taxol 10mg/kg treated WT or Cre+ APC fl/fl mice. The induction in mitotic arrest and apoptosis is significant only in WT vehicle versus treated, p<0.026. NT – vehicle-treated. (e) Representative H+E staining of crypts from Cre+ APC fl/fl mice treated with Taxol 10mg/kg and sacrificed at 3h, 6h, 9h or 24h post-injection. Scale bar 20μm.
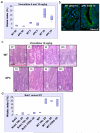
Figure 2. Resistance to Taxol in APC-deficient intestinal crypts is independent of activation of the canonical WNT/beta-catenin pathway
Mice injected with either vehicle (NT) or 10mg/kg Taxol IP (n≥3) were sacrificed 6h post-injection and the levels of mitotic arrest and apoptosis in intestinal crypts were scored by H+E staining. Mitotic index was expressed as percentage of cells per crypt. (a) IHC detecting beta-catenin showing nuclear localisation in Cre+ APC fl/fl (vehicle or Taxol 10mg/kg treated), Cre+ APC fl/fl MYC fl/fl (10mg/kg Taxol treated) and Cre+ GSK3 alpha fl/fl beta fl/fl mice. Note non-nuclear localisation of beta-catenin in WT vehicle or Taxol 10mg/kg treated intestine. (b) Mitotic index in intestinal crypts from Taxol 10mg/kg or vehicle treated WT, Cre+ MYC fl/fl, Cre+ APC fl/fl, or Cre+ APC fl/fl MYC fl/fl mice. There is no significant difference between the response in Cre+ APC fl/fl and Cre+ APC fl/fl MYC fl/fl mice and between WT and Cre+ MYC fl/fl mice, p>0.05. (c) Representative H+E staining of crypts from Cre+ MYC fl/fl (Taxol 10mg/kg) and Cre+ APC fl/fl MYC fl/fl mice (Taxol 10mg/kg). (d) Mitotic index in intestinal crypts from vehicle or Taxol 10mg/kg treated WT and Cre+ GSK3 alpha fl/fl beta fl/fl mice. (e) Representative H+E staining of crypts from Cre+ APC fl/fl, or Cre+ GSK3 alpha fl/fl beta fl/fl mice treated with Taxol 10mg/kg.
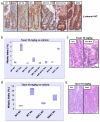
Figure 3. Resistance to Taxol in APC-deficient intestinal crypts can be reversed by increasing drug concentration
Mice injected with either vehicle (NT), or 20mg/kg Taxol IP (n≥3) were sacrificed 6h post-injection and the levels of mitotic arrest and apoptosis in intestinal crypts were scored by H+E staining. Mitotic and apoptotic index was expressed as percentage of cells per crypt. (a) and (b) Mitotic and apoptotic index in intestinal crypts from vehicle or Taxol 20mg/kg treated WT or Cre+ APC fl/fl mice. The induction in mitotic arrest is significant in both WT and Cre_+ APC fl/fl_ drug treated compared to vehicle same genotype, p<0.026. The induction in apoptosis is significant only in WT treated versus vehicle with p<0.026. (c) Representative H+E staining of crypts from Cre+ APC fl/fl mice treated with Taxol 20mg/kg and sacrificed at 3h, 6h, 9h or 24h post-injection. NT – vehicle-treated. Arrows indicate mitotic figures as a result of Taxol treatment. (d) Tubulin immunofluorescence (green) was performed on PFA-fixed tissue from WT or Cre+ APC fl/fl mice treated with vehicle (NT), 10 or 20mg/kg Taxol and taken 6h post-injection. Slides were counterstained with DAPI (blue). White arrows indicate bundled microtubules in response to Taxol treatment. Orange arrows indicate normal mitotic figures.
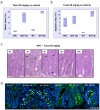
Figure 4. APC is not required as a general mitotic spindle checkpoint protein
Mice injected with either vehicle (NT), 5 or 10mg/kg Vinorelbine IV (n≥3) were sacrificed 6h post-injection and the levels of mitotic arrest and apoptosis in intestinal crypts were scored by H+E staining. Mitotic and apoptotic index was expressed as percentage of cells per crypt. (a) Mitotic index in intestinal crypts from WT or Cre+ APC fl/fl mice treated with vehicle, 5 or 10mg/kg Vinorelbine. The induction in mitosis is significant for both 5 and 10mg/kg Vinorelbine treated versus untreated p<0.026. (b) Tubulin immunofluorescence (green) was performed on PFA-fixed tissue from WT or Cre+ APC fl/fl mice treated with 10mg/kg Vinorelbine and taken 6h post-injection. Slides were counterstained with DAPI (blue). (c) Representative H+E staining of crypts from WT or Cre+ APC fl/fl mice treated with Vinorelbine 10mg/kg and sacrificed at 3h, 6h or 24h post-injection. NT – vehicle-treated. Scale bar 20μm. (d) Deletion of the bona fide mitotic spindle checkpoint protein Bub1 (Cre+ Bub1 fl/fl mice) leads to complete abrogation of mitotic arrest in intestinal crypts following treatment with either 10, 20mg/kg Taxol or 10mg/kg Vinorelbine.
Figure 5. High Taxol concentrations dramatically reduce tumour number and size in benign and invasive mouse models of intestinal cancer
Cohorts of APCMIN/+ mice (45 days of age, n≥16) were treated every 2 weeks with either 10, 20mg/kg Taxol or vehicle until they reached 100 days of age. The most efficient treatment, 20mg/kg, was then increased to 3 injections a week every other week (Txl 20 x3). The same regime was used in an invasive model of tumourigenesis using Cre+ APCfl/+ PTEN fl/fl mice. Mice started treatment 2 weeks after induction and were sacrificed at 60 days post-induction. (a) Tumour number is not decreased in APCMIN/+ mice treated with Taxol 10mg/kg once every 2 weeks (p=0.48). (b) Taxol 20mg/kg treatment (once every 2 weeks) reduces significantly the number of tumours in APCMIN/+ (p=0.0007). (c) Taxol 20mg/kg administered 3 times a week every 2 weeks further reduces the number of tumours in APCMIN/+ (p=0.0001). (d) All Taxol regimes reduce the average tumour size in APCMIN/+ mice. Vehicle versus 10/mg/kg p=0.015, vehicle versus 20mg/kg p≤0.006. (e) Taxol reduces tumour number in Cre+ APCfl/+ PTEN fl/fl mice, p=0.001.
Similar articles
- The microtubule poison vinorelbine kills cells independently of mitotic arrest and targets cells lacking the APC tumour suppressor more effectively.
Klotz DM, Nelson SA, Kroboth K, Newton IP, Radulescu S, Ridgway RA, Sansom OJ, Appleton PL, Näthke IS. Klotz DM, et al. J Cell Sci. 2012 Feb 15;125(Pt 4):887-95. doi: 10.1242/jcs.091843. Epub 2012 Mar 7. J Cell Sci. 2012. PMID: 22399804 Free PMC article. - PLK1 has tumor-suppressive potential in APC-truncated colon cancer cells.
Raab M, Sanhaji M, Matthess Y, Hörlin A, Lorenz I, Dötsch C, Habbe N, Waidmann O, Kurunci-Csacsko E, Firestein R, Becker S, Strebhardt K. Raab M, et al. Nat Commun. 2018 Mar 16;9(1):1106. doi: 10.1038/s41467-018-03494-4. Nat Commun. 2018. PMID: 29549256 Free PMC article. - Bub1 and aurora B cooperate to maintain BubR1-mediated inhibition of APC/CCdc20.
Morrow CJ, Tighe A, Johnson VL, Scott MI, Ditchfield C, Taylor SS. Morrow CJ, et al. J Cell Sci. 2005 Aug 15;118(Pt 16):3639-52. doi: 10.1242/jcs.02487. Epub 2005 Jul 26. J Cell Sci. 2005. PMID: 16046481 - The role of APC in mitosis and in chromosome instability.
Caldwell CM, Kaplan KB. Caldwell CM, et al. Adv Exp Med Biol. 2009;656:51-64. doi: 10.1007/978-1-4419-1145-2_5. Adv Exp Med Biol. 2009. PMID: 19928352 Review. - Functions of the APC tumor suppressor protein dependent and independent of canonical WNT signaling: implications for therapeutic targeting.
Hankey W, Frankel WL, Groden J. Hankey W, et al. Cancer Metastasis Rev. 2018 Mar;37(1):159-172. doi: 10.1007/s10555-017-9725-6. Cancer Metastasis Rev. 2018. PMID: 29318445 Free PMC article. Review.
Cited by
- More than two decades of Apc modeling in rodents.
Zeineldin M, Neufeld KL. Zeineldin M, et al. Biochim Biophys Acta. 2013 Aug;1836(1):80-9. doi: 10.1016/j.bbcan.2013.01.001. Epub 2013 Jan 17. Biochim Biophys Acta. 2013. PMID: 23333833 Free PMC article. Review. - Cell-cycle synchronization reverses Taxol resistance of human ovarian cancer cell lines.
Wang X, Pan L, Mao N, Sun L, Qin X, Yin J. Wang X, et al. Cancer Cell Int. 2013 Jul 30;13(1):77. doi: 10.1186/1475-2867-13-77. Cancer Cell Int. 2013. PMID: 23899403 Free PMC article. - MYC Is a Major Determinant of Mitotic Cell Fate.
Topham C, Tighe A, Ly P, Bennett A, Sloss O, Nelson L, Ridgway RA, Huels D, Littler S, Schandl C, Sun Y, Bechi B, Procter DJ, Sansom OJ, Cleveland DW, Taylor SS. Topham C, et al. Cancer Cell. 2015 Jul 13;28(1):129-40. doi: 10.1016/j.ccell.2015.06.001. Cancer Cell. 2015. PMID: 26175417 Free PMC article. - The APC tumor suppressor is required for epithelial cell polarization and three-dimensional morphogenesis.
Lesko AC, Goss KH, Yang FF, Schwertner A, Hulur I, Onel K, Prosperi JR. Lesko AC, et al. Biochim Biophys Acta. 2015 Mar;1853(3):711-23. doi: 10.1016/j.bbamcr.2014.12.036. Epub 2015 Jan 8. Biochim Biophys Acta. 2015. PMID: 25578398 Free PMC article. - MicroRNAs: key players of taxane resistance and their therapeutic potential in human cancers.
Cui SY, Wang R, Chen LB. Cui SY, et al. J Cell Mol Med. 2013 Oct;17(10):1207-17. doi: 10.1111/jcmm.12131. Epub 2013 Sep 23. J Cell Mol Med. 2013. PMID: 24106980 Free PMC article. Review.
References
- Abal M, Souto AA, Amat-Guerri F, Acuna AU, Andreu JM, Barasoain I. Centrosome and spindle pole microtubules are main targets of a fluorescent taxoid inducing cell death. Cell Motil Cytoskeleton. 2001;49:1–15. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- A11243/CRUK_/Cancer Research UK/United Kingdom
- 74711/CAPMC/ CIHR/Canada
- A7130/CRUK_/Cancer Research UK/United Kingdom
- 11243/CRUK_/Cancer Research UK/United Kingdom
- 12481/CRUK_/Cancer Research UK/United Kingdom
- 11913/CRUK_/Cancer Research UK/United Kingdom
- 12858/CAPMC/ CIHR/Canada
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials