Interleukin-23 drives intestinal inflammation through direct activity on T cells - PubMed (original) (raw)
Interleukin-23 drives intestinal inflammation through direct activity on T cells
Philip P Ahern et al. Immunity. 2010.
Erratum in
- Immunity. 2011 Mar 25;34(3):448
Abstract
Mutations in the IL23R gene are linked to inflammatory bowel disease susceptibility. Experimental models have shown that interleukin-23 (IL-23) orchestrates innate and T cell-dependent colitis; however, the cell populations it acts on to induce intestinal immune pathology are unknown. Here, using Il23r(-/-) T cells, we demonstrated that T cell reactivity to IL-23 was critical for development of intestinal pathology, but not for systemic inflammation. Through direct signaling into T cells, IL-23 drove intestinal T cell proliferation, promoted intestinal Th17 cell accumulation, and enhanced the emergence of an IL-17A(+)IFN-gamma(+) population of T cells. Furthermore, IL-23R signaling in intestinal T cells suppressed the differentiation of Foxp3(+) cells and T cell IL-10 production. Although Il23r(-/-) T cells displayed unimpaired Th1 cell differentiation, these cells showed impaired proliferation and failed to accumulate in the intestine. Together, these results highlight the multiple functions of IL-23 signaling in T cells that contribute to its colitogenic activity.
Copyright 2010 Elsevier Inc. All rights reserved.
Figures
Graphical abstract
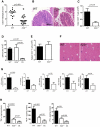
Figure 1
IL-23R Expression on T Cells Is Required for Intestinal but Not Systemic Inflammation C57BL.6.Rag1−/− mice were transferred with 4 × 105 CD4+CD45RBhi T cells from WT or Il23r−/− donors. Mice were sacrificed when recipients of WT T cells developed clinical signs of disease (∼9 weeks after transfer) and assessed for intestinal and systemic inflammation. (A) Colitis scores. (B) Representative photomicrographs of mid colon sections. (C) Total CD4+ T cells in colon. (D) Total splenocytes. (E) Total CD4+ T cells in spleen. (F) Representative photomicrographs of liver sections. (G) Expression of cytokine mRNA in colon tissue homogenates, normalized to Hprt. (H) Concentration of cytokines in colon tissue homogenates, normalized to total protein. Data represent pooled results from two to three independent experiments. Bars represent the mean, error bars represent the SEM, and each symbol represents an individual mouse, n = 11–16 (WT T cells), n = 13–18 (Il23r−/− T cells), n = 3–13 (Controls; Ctl.). Statistical significance was determined with the Mann-Whitney test.
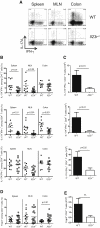
Figure 2
IL-23 Regulates Intestinal Th Cell Responses via Direct Effects on T Cells C57BL.6.Rag1−/− mice were transferred with 4 × 105 CD4+CD45RBhi T cells from WT or Il23r−/− donors and sacrificed when recipients of WT T cells developed clinical signs of disease (∼9 weeks after transfer). IL-17A, IFN-γ, and Foxp3 amounts in T cells from various tissues were assessed by intracellular flow cytometry after in vitro restimulation with PMA and ionomycin. (A) Representative flow cytometry plots of CD4+ T cells from the spleen, MLN, and colon. Numbers in quadrants represent frequencies. (B) Frequencies of IL-17A+ and/or IFN-γ+ T cells in the spleen, MLN, and colon. (C) Total numbers of IL-17A+ and/or IFN-γ+ CD4+ T cells in the colon. (D) Frequencies of Foxp3+ CD4+ T cells in the spleen, MLN, and colon. (E) Total number of Foxp3+ CD4+ T cells in the colon. Data represent pooled results from two to three independent experiments, bars represent the mean, error bars represent the SEM, and each symbol represents an individual mouse. Statistical significance was determined with the Mann-Whitney test, n = 11–16 (WT), n = 13–18 (Il23r−/−).
Figure 3
IL-23 Directly Promotes Intestinal T cell Proliferation (A and B) C57BL.6.Rag1−/− mice were transferred with 4 × 106 CFSE-labeled CD4+CD45RBhi T cells from WT or Il23r−/− donors and sacrificed 12 days after transfer. (A) Total CD4+ T cell numbers and (B) representative flow cytometry plots showing CFSE dilution profiles of T cells from the spleen and colon are presented. (C–E) C57BL.6.Rag1−/− mice were transferred with 4 × 105 CD4+CD45RBhi T cells from WT or Il23r−/− donors and sacrificed when recipients of WT T cells developed clinical signs of disease (∼6 weeks after transfer). IL-17A, IFN-γ, and Ki67 expression in CD4+ T cells from the colon were assessed by intracellular flow cytometry after in vitro restimulation with PMA and ionomycin. (C) Frequencies, (D) representative flow cytometric plots (numbers in gate represent frequencies), and (E) total numbers of IL-17A+IFN-γ−Ki-67+, IL-17A+IFN-γ+Ki-67+, and IL-17A−IFN-γ+Ki-67+ CD4+ T cells in the colon. Data represent pooled results from two independent experiments (A and B) or of a single experiment (C–E), bars represent the mean, error bars represent the SEM, and each symbol represents an individual mouse. Statistical significance was determined with the Mann-Whitney test, n = 4-11 (WT), n = 5-11 (Il23r−/−).
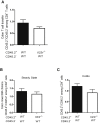
Figure 4
IL-23 Controls T cell Accumulation in the Intestine via a Cell-Extrinsic Mechanism C57BL.6.Rag1−/− mice were transferred with 1:1 mixtures of CD45.2− (WT) + CD45.2+ (WT) or CD45.2− (WT) + CD45.2+(Il23r−/−) CD4+CD45RBhi T cells. Mice were sacrificed upon development of clinical signs of inflammation (∼8 weeks) and populations of T cells were identified on the basis of the expression of CD45.2. (A) Ratio of CD45.2+/CD45.2− CD4+ T cells in colon. Data represent pooled results from two independent experiments; n = 15 (WT + WT), n = 12 (WT + Il23r−/−). Bars represent the mean ± SEM. (B and C) Sublethally irradiated C57BL.6.Rag1−/− mice were reconstituted with 1:1 mixtures of CD45.2− (WT) + CD45.2+ (WT) or CD45.2− (WT) + CD45.2+(Il23r−/−) bone marrow cells. Ratio of CD45.2+/CD45.2− T cells recovered from the colon during steady state (B) or during colitis induced by infection with Helicobacter hepaticus plus treatment with a blocking IL-10R mAb (C). Data represent results from a single experiment; n = 5–7 (WT + WT), n = 5–7 (WT + Il23r−/− T cells). Bars represent the mean ± SEM.
Figure 5
IL-23 Modulates Th17 Cell Phenotype via a Direct Cell-Intrinsic Mechanism C57BL.6.Rag1−/− mice were transferred with 1:1 mixtures of CD45.2− (WT) + CD45.2+ (WT), or CD45.2− (WT) + CD45.2+(Il23r−/−) CD4+CD45RBhi T cells. Mice were sacrificed upon development of clinical signs of inflammation (∼8 weeks) and IL-17A and IFN-γ levels in colonic CD4+ T cells were assessed by intracellular flow cytometry after in vitro restimulation with PMA and ionomycin. (A) Representative flow cytometry plots of CD4+ T cells isolated from the colon; numbers in quadrants represent frequencies. (B–D) Frequencies of IL-17A+ and/or IFN-γ+ CD4+ T cells in the colon of cotransferred mice. Data represent pooled results from two independent experiments. Bars represent the mean; each symbol represents an individual mouse. Statistical significance was determined with the Mann-Whitney test, n = 12 (WT + WT), n = 10 (WT + Il23r−/−).
Figure 6
IL-23 Inhibits Intestinal iTreg Cell Generation via a Direct Cell-Intrinsic Mechanism (A and B) C57BL.6.Rag1−/− mice were transferred with 1:1 mixtures of CD45.2− (WT) + CD45.2+ (WT) or CD45.2− (WT) + CD45.2+(Il23r−/−) CD4+CD45RBhi T cells. Mice were sacrificed upon development of clinical signs of inflammation (∼8 weeks). (A) The frequency of Foxp3+ cells among T cells in the colon was assessed by intracellular flow cytometry. (B) Representative FACS plots showing Foxp3 expression in CD4+ T cells in the colon; numbers in quadrants represent frequencies. Data represent pooled results from two independent experiments, n = 15 (WT + WT), n = 12 (WT + Il23r−/−). (C and D) Sublethally irradiated C57BL.6.Rag1−/− mice were reconstituted with 1:1 mixtures of CD45.2− (WT) + CD45.2+ (WT) or CD45.2− (WT) + CD45.2+(Il23r−/−) bone marrow cells. The frequency of Foxp3+ cells among CD4+ cells in the colon was assessed by intracellular FACS during steady state (C) or during colitis induced by infection with Helicobacter hepaticus plus treatment with a blocking IL-10R mAb (D). Data represent results from a single experiment; n = 5–7 (WT + WT), n = 5–7 (WT + Il23r−/− T cells). (E) Colitis scores from mice in (A); data represent pooled results from two independent experiments. (F) Il10 mRNA levels and protein levels in supernatants after restimulation of CD4+ T cells purified from the colons of C57BL.6.Rag1−/− mice transferred with WT or Il23r−/− CD4+CD45RBhi T cells as described in Figure 1 (single transfer) or from recipients of 1:1 mixtures of WT + Il23r−/− CD4+CD45RBhi T cells as described above (cotransfer). Data represent pooled results from two independent experiments (top, n = 7–10 per group) or from a single experiment (bottom, n = 5 per group). Bars represent the mean; each symbol represents an individual mouse; statistical significance was determined with the Mann-Whitney test.
Comment in
- Checks and balances: IL-23 in the intestine.
Huber S, Flavell RA. Huber S, et al. Immunity. 2010 Aug 27;33(2):150-2. doi: 10.1016/j.immuni.2010.08.005. Immunity. 2010. PMID: 20732638 Free PMC article.
Similar articles
- Th17 cells induce colitis and promote Th1 cell responses through IL-17 induction of innate IL-12 and IL-23 production.
Feng T, Qin H, Wang L, Benveniste EN, Elson CO, Cong Y. Feng T, et al. J Immunol. 2011 Jun 1;186(11):6313-8. doi: 10.4049/jimmunol.1001454. Epub 2011 Apr 29. J Immunol. 2011. PMID: 21531892 Free PMC article. - Innate lymphoid cells drive interleukin-23-dependent innate intestinal pathology.
Buonocore S, Ahern PP, Uhlig HH, Ivanov II, Littman DR, Maloy KJ, Powrie F. Buonocore S, et al. Nature. 2010 Apr 29;464(7293):1371-5. doi: 10.1038/nature08949. Nature. 2010. PMID: 20393462 Free PMC article. - Elevated IL-23R Expression and Foxp3+Rorgt+ Cells in Intestinal Mucosa During Acute and Chronic Colitis.
Yang J, Xu L. Yang J, et al. Med Sci Monit. 2016 Aug 8;22:2785-92. doi: 10.12659/msm.896827. Med Sci Monit. 2016. PMID: 27498708 Free PMC article. - Interleukin-23 and T helper 17-type responses in intestinal inflammation: from cytokines to T-cell plasticity.
Morrison PJ, Ballantyne SJ, Kullberg MC. Morrison PJ, et al. Immunology. 2011 Aug;133(4):397-408. doi: 10.1111/j.1365-2567.2011.03454.x. Epub 2011 Jun 2. Immunology. 2011. PMID: 21631495 Free PMC article. Review. - IL-23 in inflammatory bowel diseases and colon cancer.
Neurath MF. Neurath MF. Cytokine Growth Factor Rev. 2019 Feb;45:1-8. doi: 10.1016/j.cytogfr.2018.12.002. Epub 2018 Dec 12. Cytokine Growth Factor Rev. 2019. PMID: 30563755 Review.
Cited by
- Experimental Models of Inflammatory Bowel Diseases.
Kiesler P, Fuss IJ, Strober W. Kiesler P, et al. Cell Mol Gastroenterol Hepatol. 2015 Mar 1;1(2):154-170. doi: 10.1016/j.jcmgh.2015.01.006. Cell Mol Gastroenterol Hepatol. 2015. PMID: 26000334 Free PMC article. - Decoding IL-23 Signaling Cascade for New Therapeutic Opportunities.
Pastor-Fernández G, Mariblanca IR, Navarro MN. Pastor-Fernández G, et al. Cells. 2020 Sep 7;9(9):2044. doi: 10.3390/cells9092044. Cells. 2020. PMID: 32906785 Free PMC article. Review. - Myocarditis and inflammatory cardiomyopathy: current evidence and future directions.
Tschöpe C, Ammirati E, Bozkurt B, Caforio ALP, Cooper LT, Felix SB, Hare JM, Heidecker B, Heymans S, Hübner N, Kelle S, Klingel K, Maatz H, Parwani AS, Spillmann F, Starling RC, Tsutsui H, Seferovic P, Van Linthout S. Tschöpe C, et al. Nat Rev Cardiol. 2021 Mar;18(3):169-193. doi: 10.1038/s41569-020-00435-x. Epub 2020 Oct 12. Nat Rev Cardiol. 2021. PMID: 33046850 Free PMC article. Review. - Prostaglandin E2-induced IL-23p19 subunit is regulated by cAMP-responsive element-binding protein and C/AATT enhancer-binding protein β in bone marrow-derived dendritic cells.
Kocieda VP, Adhikary S, Emig F, Yen JH, Toscano MG, Ganea D. Kocieda VP, et al. J Biol Chem. 2012 Oct 26;287(44):36922-35. doi: 10.1074/jbc.M112.402958. Epub 2012 Sep 12. J Biol Chem. 2012. PMID: 22977257 Free PMC article. - Th17 cells give rise to Th1 cells that are required for the pathogenesis of colitis.
Harbour SN, Maynard CL, Zindl CL, Schoeb TR, Weaver CT. Harbour SN, et al. Proc Natl Acad Sci U S A. 2015 Jun 2;112(22):7061-6. doi: 10.1073/pnas.1415675112. Epub 2015 May 18. Proc Natl Acad Sci U S A. 2015. PMID: 26038559 Free PMC article.
References
- Ahern P.P., Izcue A., Maloy K.J., Powrie F. The interleukin-23 axis in intestinal inflammation. Immunol. Rev. 2008;226:147–159. - PubMed
- Bettelli E., Carrier Y., Gao W., Korn T., Strom T.B., Oukka M., Weiner H.L., Kuchroo V.K. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases