Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network - PubMed (original) (raw)
. 2010 Sep;126(3):443-56.
doi: 10.1542/peds.2009-2959. Epub 2010 Aug 23.
Nellie I Hansen, Edward F Bell, Seetha Shankaran, Abbot R Laptook, Michele C Walsh, Ellen C Hale, Nancy S Newman, Kurt Schibler, Waldemar A Carlo, Kathleen A Kennedy, Brenda B Poindexter, Neil N Finer, Richard A Ehrenkranz, Shahnaz Duara, Pablo J Sánchez, T Michael O'Shea, Ronald N Goldberg, Krisa P Van Meurs, Roger G Faix, Dale L Phelps, Ivan D Frantz 3rd, Kristi L Watterberg, Shampa Saha, Abhik Das, Rosemary D Higgins; Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network
Collaborators, Affiliations
- PMID: 20732945
- PMCID: PMC2982806
- DOI: 10.1542/peds.2009-2959
Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network
Barbara J Stoll et al. Pediatrics. 2010 Sep.
Abstract
Objective: This report presents data from the Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network on care of and morbidity and mortality rates for very low birth weight infants, according to gestational age (GA).
Methods: Perinatal/neonatal data were collected for 9575 infants of extremely low GA (22-28 weeks) and very low birth weight (401-1500 g) who were born at network centers between January 1, 2003, and December 31, 2007.
Results: Rates of survival to discharge increased with increasing GA (6% at 22 weeks and 92% at 28 weeks); 1060 infants died at <or=12 hours, with most early deaths occurring at 22 and 23 weeks (85% and 43%, respectively). Rates of prenatal steroid use (13% and 53%, respectively), cesarean section (7% and 24%, respectively), and delivery room intubation (19% and 68%, respectively) increased markedly between 22 and 23 weeks. Infants at the lowest GAs were at greatest risk for morbidities. Overall, 93% had respiratory distress syndrome, 46% patent ductus arteriosus, 16% severe intraventricular hemorrhage, 11% necrotizing enterocolitis, and 36% late-onset sepsis. The new severity-based definition of bronchopulmonary dysplasia classified more infants as having bronchopulmonary dysplasia than did the traditional definition of supplemental oxygen use at 36 weeks (68%, compared with 42%). More than one-half of infants with extremely low GAs had undetermined retinopathy status at the time of discharge. Center differences in management and outcomes were identified.
Conclusion: Although the majority of infants with GAs of >or=24 weeks survive, high rates of morbidity among survivors continue to be observed.
Figures
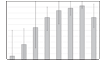
FIGURE 1
Survival to discharge according to GA among 9575 VLBW infants born in NICHD NRN centers between January 1, 2003, and December 31, 2007. The thin lines indicate ranges across centers.
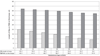
FIGURE 2
Median length of hospitalization (in weeks) and median PMA at discharge (in weeks) according to GA at birth among 6859 VLBW infants who were born in NICHD NRN centers between January 1, 2003, and December 31, 2007, and survived to discharge.
Similar articles
- Neonatal outcomes of extremely preterm infants from taiwan: comparison with Canada, Japan, and the USA.
Su BH, Hsieh WS, Hsu CH, Chang JH, Lien R, Lin CH; Premature Baby Foundation of Taiwan (PBFT). Su BH, et al. Pediatr Neonatol. 2015 Feb;56(1):46-52. doi: 10.1016/j.pedneo.2014.05.002. Epub 2014 Aug 22. Pediatr Neonatol. 2015. PMID: 25154794 - Mortality, In-Hospital Morbidity, Care Practices, and 2-Year Outcomes for Extremely Preterm Infants in the US, 2013-2018.
Bell EF, Hintz SR, Hansen NI, Bann CM, Wyckoff MH, DeMauro SB, Walsh MC, Vohr BR, Stoll BJ, Carlo WA, Van Meurs KP, Rysavy MA, Patel RM, Merhar SL, Sánchez PJ, Laptook AR, Hibbs AM, Cotten CM, D'Angio CT, Winter S, Fuller J, Das A; Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network. Bell EF, et al. JAMA. 2022 Jan 18;327(3):248-263. doi: 10.1001/jama.2021.23580. JAMA. 2022. PMID: 35040888 Free PMC article. - Neonatal mortality and morbidity among infants between 24 to 31 complete weeks: a multicenter survey in China from 2013 to 2014.
Kong X, Xu F, Wu R, Wu H, Ju R, Zhao X, Tong X, Lv H, Ding Y, Liu F, Xu P, Liu W, Cheng H, Chen T, Zeng S, Jia W, Li Z, Qiu H, Wang J, Feng Z. Kong X, et al. BMC Pediatr. 2016 Nov 3;16(1):174. doi: 10.1186/s12887-016-0716-5. BMC Pediatr. 2016. PMID: 27809893 Free PMC article. - Outcome prediction in newborn infants: Past, present, and future.
Shukla VV, Rysavy MA, Das A, Tyson JE, Bell EF, Ambalavanan N, Carlo WA. Shukla VV, et al. Semin Perinatol. 2022 Nov;46(7):151641. doi: 10.1016/j.semperi.2022.151641. Epub 2022 Jun 10. Semin Perinatol. 2022. PMID: 35850743 Free PMC article. Review. - Overview of the neonatal research network: History, contributions, challenges, and future.
Watterberg KL, Carlo WA, Brion LP, Cotten CM, Higgins RD. Watterberg KL, et al. Semin Perinatol. 2022 Nov;46(7):151634. doi: 10.1016/j.semperi.2022.151634. Epub 2022 Jun 10. Semin Perinatol. 2022. PMID: 35786518 Free PMC article. Review.
Cited by
- Nintedanib as a strategy to prevent bronchopulmonary dysplasia: dosing and tim(-ing) is of the essence.
Mathias MM, Ganguly A, Tipple TE. Mathias MM, et al. Pediatr Res. 2024 Nov 11. doi: 10.1038/s41390-024-03713-3. Online ahead of print. Pediatr Res. 2024. PMID: 39528746 No abstract available. - Endoplasmic Reticulum Stress in Bronchopulmonary Dysplasia: Contributor or Consequence?
Wu TJ, Teng M, Jing X, Pritchard KA Jr, Day BW, Naylor S, Teng RJ. Wu TJ, et al. Cells. 2024 Oct 26;13(21):1774. doi: 10.3390/cells13211774. Cells. 2024. PMID: 39513884 Free PMC article. Review. - [Measurement of intrinsic positive end-expiratory pressure and clinical outcomes of infants with severe bronchopulmonary dysplasia].
Qin X, Zhao XP, Zhang HY. Qin X, et al. Zhongguo Dang Dai Er Ke Za Zhi. 2024 Oct 15;26(10):1034-1039. doi: 10.7499/j.issn.1008-8830.2404133. Zhongguo Dang Dai Er Ke Za Zhi. 2024. PMID: 39467671 Free PMC article. Chinese. - Bronchopulmonary dysplasia demonstrates dysregulated autotaxin/lysophosphatidic acid signaling in a neonatal mouse model.
Ha AW, Sudhadevi T, Jafri A, Mayer C, MacFarlane PM, Natarajan V, Harijith A. Ha AW, et al. Pediatr Res. 2024 Oct 16. doi: 10.1038/s41390-024-03610-9. Online ahead of print. Pediatr Res. 2024. PMID: 39415037 - Nintedanib preserves lung growth and prevents pulmonary hypertension in a hyperoxia-induced lung injury model.
Ding KL, Smith C, Seedorf G, Abman SH. Ding KL, et al. Pediatr Res. 2024 Oct 11. doi: 10.1038/s41390-024-03562-0. Online ahead of print. Pediatr Res. 2024. PMID: 39394424
References
- Hack M, Horbar JD, Malloy MH, Tyson JE, Wright E, Wright L. Very low birth weight outcomes of the National Institute of Child Health and Human Development Neonatal Network. Pediatrics. 1991;87(5):587–597. - PubMed
- Hack M, Wright LL, Shankaran S, et al. Very-low-birth-weight outcomes of the National Institute of Child Health and Human Development Neonatal Network, November 1989 to October 1990. Am J Obstet Gynecol. 1995;172(2):457–464. - PubMed
- Fanaroff AA, Wright LL, Stevenson DK, et al. Very-low-birth-weight outcomes of the National Institute of Child Health and Human Development Neonatal Research Network, May 1991–December 1992. Am J Obstet Gynecol. 1995;173(5):1423–1431. - PubMed
- Stevenson DK, Wright LL, Lemons JA, et al. Very low birth weight outcomes of the National Institute of Child Health and Human Development Neonatal Research Network, January 1993 through December 1994. Am J Obstet Gynecol. 1998;179(6):1632–1639. - PubMed
- Lemons JA, Bauer CR, Oh W, et al. Very low birth weight outcomes of the National Institute of Child Health and Human Development Neonatal Research Network. January 1995 through December 1996. Pediatrics. 2001;107(1) Available at: http://www.pediatrics.org/cgi/content/full/107/1/e1. - PubMed
Publication types
MeSH terms
Grants and funding
- U10 HD027851-13/HD/NICHD NIH HHS/United States
- U10 HD021385/HD/NICHD NIH HHS/United States
- U10 HD027851-16/HD/NICHD NIH HHS/United States
- U10 HD027851-15/HD/NICHD NIH HHS/United States
- U01 HD040636/HD/NICHD NIH HHS/United States
- U10 HD034216/HD/NICHD NIH HHS/United States
- U10 HD040498/HD/NICHD NIH HHS/United States
- U10 HD027856/HD/NICHD NIH HHS/United States
- U10 HD021373/HD/NICHD NIH HHS/United States
- UL1 RR024139/RR/NCRR NIH HHS/United States
- U24 HD092094/HD/NICHD NIH HHS/United States
- U10 HD053124/HD/NICHD NIH HHS/United States
- U10 HD053119/HD/NICHD NIH HHS/United States
- U01 HD036790/HD/NICHD NIH HHS/United States
- U10 HD021364/HD/NICHD NIH HHS/United States
- U10 HD027851-17/HD/NICHD NIH HHS/United States
- U10 HD027880/HD/NICHD NIH HHS/United States
- U10 HD040521/HD/NICHD NIH HHS/United States
- U10 HD027851-14/HD/NICHD NIH HHS/United States
- U10 HD053109/HD/NICHD NIH HHS/United States
- M01 RR008084/RR/NCRR NIH HHS/United States
- UL1 TR001449/TR/NCATS NIH HHS/United States
- UG1 HD053089/HD/NICHD NIH HHS/United States
- U10 HD040461/HD/NICHD NIH HHS/United States
- M01 RR016587/RR/NCRR NIH HHS/United States
- U10 HD040689/HD/NICHD NIH HHS/United States
- U10 HD040492/HD/NICHD NIH HHS/United States
- U10 HD027853/HD/NICHD NIH HHS/United States
- U10 HD027904/HD/NICHD NIH HHS/United States
- U10 HD021397/HD/NICHD NIH HHS/United States
- UL1 TR000454/TR/NCATS NIH HHS/United States
- U10 HD027871/HD/NICHD NIH HHS/United States
- UL1 TR000041/TR/NCATS NIH HHS/United States
- M01 RR007122/RR/NCRR NIH HHS/United States
- U10 HD027851/HD/NICHD NIH HHS/United States
- UG1 HD027851/HD/NICHD NIH HHS/United States
- UL1 RR025008/RR/NCRR NIH HHS/United States
- M01 RR006022/RR/NCRR NIH HHS/United States
- U10 HD053089/HD/NICHD NIH HHS/United States
- UL1 RR024979/RR/NCRR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical