Pharmacokinetics of anti-hepcidin monoclonal antibody Ab 12B9m and hepcidin in cynomolgus monkeys - PubMed (original) (raw)
Pharmacokinetics of anti-hepcidin monoclonal antibody Ab 12B9m and hepcidin in cynomolgus monkeys
Jim J Xiao et al. AAPS J. 2010 Dec.
Abstract
Hepcidin is a key regulator responsible for systemic iron homeostasis. A semi-mechanistic PK model for hepcidin and a fully human anti-hepcidin monoclonal antibody (Ab 12B9m) was developed to describe their total (free + bound) serum concentration-time data after single and multiple weekly intravenous or subcutaneous doses of Ab 12B9m. The model was based on target mediated drug disposition and the IgG-FcRn interaction concepts published previously. Both total Ab 12B9m and total hepcidin exhibited nonlinear kinetics due to saturable Fc-FcRn interaction. Ab 12B9m showed a limited volume of distribution and negligible linear elimination from serum. The nonlinear elimination of Ab 12B9m was attributed to the endosomal degradation of Ab 12B9m that was not bound to the FcRn receptor. The terminal half-life, assumed to be the same for free and total serum Ab 12B9m, was estimated to be 16.5 days. The subcutaneous absorption of Ab 12B9m was described with a first-order absorption rate constant k(a) of 0.0278 h⁻¹, with 86% bioavailability. The model suggested a rapid hepcidin clearance of approximately 800 mL h⁻¹ kg⁻¹. Only the highest-tested Ab 12B9m dose of 300 mg kg⁻¹ week⁻¹ was able to maintain free hepcidin level below the baseline during the dosing intervals. Free Ab 12B9m and free hepcidin concentrations were simulated, and their PK profiles were nonlinear as affected by their binding to each other. Additionally, the total amount of FcRn receptor involved in Ab 12B9m recycling at a given time was calculated empirically, and the temporal changes in the free FcRn levels upon Ab 12B9m administration were inferred.
Figures
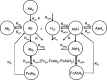
Fig. 1
Semi-mechanistic pharmacokinetic model for H25 and Ab 12B9m in cynomolgus monkeys. Absc represents the SC depot for Ab 12B9m SC dosing. Ab 12B9m and Ab 12B9m–H25 complex distribute into their central compartments (Ab and AbH), peripheral compartments (Abp and AbHp), and endosome compartments (AbE and AbHE). In endosome, Ab 12B9m and Ab 12B9m–H25 complex binds to FcRn to form complexes (FcAbE and FcAbHE). The intercompartment distribution was described with a set of first-order rate constants (k cp, k pc, k up, and k R), and elimination from Ab and AbH follows first-order kinetics (k). It was assumed that Ab 12B9m and Ab 12B9m–H25 complex share the same parameter as listed above. H25 is produced at a constant rate (k inH) and eliminated with first-order kinetics (k H). In serum, the binding between Ab 12B9m and H25 is governed by the association rate constant (k onH) and the dissociation rate constant (K offH); similarly, in endosome, the binding of Ab 12B9m and Ab 12B9m–H25 complex to FcRn is described by k onA and k offA
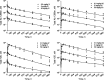
Fig. 2
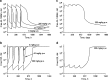
Time course of total Ab 12B9m (upper panels) and H25 (lower panels) concentrations following repeated weekly intravenous (left panels) or subcutaneous (right panels) administrations of Ab 12B9m. The symbols represent individual measured concentrations, and the solid lines represent model predictions
Fig. 3
Time course of total Ab 12B9m (upper panels) and H25 (lower panels) concentrations following single intravenous (left panels) or subcutaneous (right panels) administration. The symbols represent individual measured concentrations, and the solid lines represent model predictions
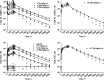
Fig. 4
Simulation of free Ab 12B9m and free H25 (solid lines) as well as Ab 12B9m–H25 complex (broken lines) serum concentrations following single IV and SC administration of Ab 12B9m. Parameter values used for simulations are presented in Table I. a Free Ab 12B9m and Ab 12B9m–H25 complex profiles after an IV dose; b free H25 profiles after an IV dose; c free Ab 12B9m and Ab 12B9m–H25 complex profiles after a SC dose; d free H25 profiles after a SC dose
Fig. 5
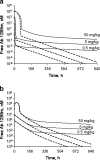
Simulation of time courses of free Ab 12B9m (upper panels) and free H25 (lower panels) concentrations following Ab 12B9m multiple weekly intravenous (left panels) and subcutaneous (right panels) administration. Parameter values used for simulations are presented in Table I. a Free Ab 12B9m and Ab 12B9m–H25 complex profiles after IV doses; b free H25 profiles after IV doses; c free Ab 12B9m and Ab 12B9m–H25 complex profiles after SC doses; d free H25 profiles after SC doses
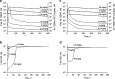
Fig. 6
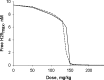
Plot of the maximum of free H25 serum concentration at steady state as a function of dose administered once a week. The solid line represents IV injections, and the broken line represents SC injections. Parameter values used for simulations are presented in Table I
Fig. 7
Simulations of free Ab 12B9m serum concentrations following a single IV (a) and SC (b) dose of Ab 12B9m in the presence (solid lines) and absence (broken lines) of the binding to the FcRn receptor. Parameter values used for simulations are presented in Table I. The broken lines were generated assuming Fctot = 0
Fig. 8
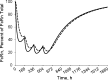
Simulation of free FcRn concentrations as percent of the total FcRn concentrations upon administration of multiple IV (solid lines) and SC (broken lines) dose 300 mg/kg q.w. of Ab 12B9m. The percentage was calculated according to Eq. 22. Parameter values used for simulations are presented in Table I
Similar articles
- Pharmacodynamic Model of Hepcidin Regulation of Iron Homeostasis in Cynomolgus Monkeys.
Krzyzanski W, Xiao JJ, Sasu B, Hinkle B, Perez-Ruixo JJ. Krzyzanski W, et al. AAPS J. 2016 May;18(3):713-27. doi: 10.1208/s12248-016-9886-1. Epub 2016 Feb 25. AAPS J. 2016. PMID: 26917226 Free PMC article. - Modification of the Fc Region of a Human Anti-oncostatin M Monoclonal Antibody for Higher Affinity to FcRn Receptor and Extension of Half-life in Cynomolgus Monkeys.
Nnane IP, Han C, Jiao Q, Tam SH, Davis HM, Xu Z. Nnane IP, et al. Basic Clin Pharmacol Toxicol. 2017 Jul;121(1):13-21. doi: 10.1111/bcpt.12761. Epub 2017 Mar 8. Basic Clin Pharmacol Toxicol. 2017. PMID: 28132416 - Are endosomal trafficking parameters better targets for improving mAb pharmacokinetics than FcRn binding affinity?
Gurbaxani B, Dostalek M, Gardner I. Gurbaxani B, et al. Mol Immunol. 2013 Dec;56(4):660-74. doi: 10.1016/j.molimm.2013.05.008. Epub 2013 Aug 2. Mol Immunol. 2013. PMID: 23917469 Review. - Regulation of iron metabolism by hepcidin.
Nemeth E, Ganz T. Nemeth E, et al. Annu Rev Nutr. 2006;26:323-42. doi: 10.1146/annurev.nutr.26.061505.111303. Annu Rev Nutr. 2006. PMID: 16848710 Review.
Cited by
- Pharmacokinetics and Pharmacodynamics of Rusfertide, a Hepcidin Mimetic, Following Subcutaneous Administration of a Lyophilized Powder Formulation in Healthy Volunteers.
Modi NB, Khanna S, Rudraraju S, Valone F. Modi NB, et al. Drugs R D. 2024 Nov 15. doi: 10.1007/s40268-024-00497-z. Online ahead of print. Drugs R D. 2024. PMID: 39546273 - Effect of Different Dietary Iron Contents on Liver Transcriptome Characteristics in Wujin Pigs.
Gao L, Xing X, Guo R, Li Q, Xu Y, Pan H, Ji P, Wang P, Yu C, Li J, An Q. Gao L, et al. Animals (Basel). 2024 Aug 19;14(16):2399. doi: 10.3390/ani14162399. Animals (Basel). 2024. PMID: 39199933 Free PMC article. - Effects of green tea polyphenols on inflammation and iron status.
Jorgenson MC, Aguree S, Schalinske KL, Reddy MB. Jorgenson MC, et al. J Nutr Sci. 2023 Nov 30;12:e119. doi: 10.1017/jns.2023.107. eCollection 2023. J Nutr Sci. 2023. PMID: 38155809 Free PMC article. - Extracellular targeted protein degradation: an emerging modality for drug discovery.
Wells JA, Kumru K. Wells JA, et al. Nat Rev Drug Discov. 2024 Feb;23(2):126-140. doi: 10.1038/s41573-023-00833-z. Epub 2023 Dec 7. Nat Rev Drug Discov. 2024. PMID: 38062152 Review. - Mechanisms of Obesity-Induced Changes in Pharmacokinetics of IgG in Rats.
Gao X, Sheng YH, Yu S, Li J, Rosa R, Girgis S, Guo T, Brunetti L, Kagan L. Gao X, et al. Pharm Res. 2023 May;40(5):1223-1238. doi: 10.1007/s11095-023-03496-y. Epub 2023 Mar 22. Pharm Res. 2023. PMID: 36949370 Free PMC article.
References
- Donovan A, Roy CN, Andrews NC. The ins and outs of iron homeostasis. Physiology (Bethesda) 2006;21:115–123. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources