Microarray analysis of iris gene expression in mice with mutations influencing pigmentation - PubMed (original) (raw)
Microarray analysis of iris gene expression in mice with mutations influencing pigmentation
Colleen M Trantow et al. Invest Ophthalmol Vis Sci. 2011.
Abstract
Purpose: Several ocular diseases involve the iris, notably including oculocutaneous albinism, pigment dispersion syndrome, and exfoliation syndrome. To screen for candidate genes that may contribute to the pathogenesis of these diseases, genome-wide iris gene expression patterns were comparatively analyzed from mouse models of these conditions.
Methods: Iris samples from albino mice with a Tyr mutation, pigment dispersion-prone mice with Tyrp1 and Gpnmb mutations, and mice resembling exfoliation syndrome with a Lyst mutation were compared with samples from wild-type mice. All mice were strain (C57BL/6J), age (60 days old), and sex (female) matched. Microarrays were used to compare transcriptional profiles, and differentially expressed transcripts were described by functional annotation clustering using DAVID Bioinformatics Resources. Quantitative real-time PCR was performed to validate a subset of identified changes.
Results: Compared with wild-type C57BL/6J mice, each disease context exhibited a large number of statistically significant changes in gene expression, including 685 transcripts differentially expressed in albino irides, 403 in pigment dispersion-prone irides, and 460 in exfoliative-like irides.
Conclusions: Functional annotation clusterings were particularly striking among the overrepresented genes, with albino and pigment dispersion-prone irides both exhibiting overall evidence of crystallin-mediated stress responses. Exfoliative-like irides from mice with a Lyst mutation showed overall evidence of involvement of genes that influence immune system processes, lytic vacuoles, and lysosomes. These findings have several biologically relevant implications, particularly with respect to secondary forms of glaucoma, and represent a useful resource as a hypothesis-generating dataset.
Figures
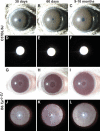
Figure 1.
Iris phenotypes of wild-type C57BL/6J and albino B6.Tyrc-2J mice. Slit lamp images of eyes with broad-beam (rows 1, 3) and transilluminating (rows 2, 4) light. (A–C) At all ages, wild-type C57BL/6J irides had a smooth-appearing surface accentuated by numerous underlying vessels and a uniformly deep sienna-brown color. (D–F) With transilluminating illumination, C57BL/6J irides appeared black at all ages, indicating an intact healthy iris (the bright white circle is a reflection of the photographic flash and not an iris defect). (G–I) At all ages, B6.Tyrc-2J irides had a complete lack of melanin pigment, but otherwise remained intact. (J–L) With transilluminating illumination, B6.Tyrc-2J irides freely passed light across most areas. Because it is not transparent, the iridial vasculature was prominently visible.
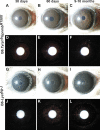
Figure 2.
Iris phenotypes of pigment dispersion–prone B6.Tyrp1b GpnmbR150X and exfoliative-like B6-Lystbg-J mice. Slit lamp images of eyes with broad beam (rows 1, 3) and transilluminating (rows 2, 4) light. (A, B) Through 5 months of age, the irides of B6.Tyrp1b GpnmbR150X mice were very similar to wild-type. (C) With increasing age, the pigment-dispersing iris disease in B6.Tyrp1b GpnmbR150X mice was evident by the presence of dispersed pigment across the iris, giving it a granular appearance, and within the pupil. (D, E) With transilluminating light, B6.Tyrp1b GpnmbR150X irides from young mice showed mild transillumination defects (red areas). (F) With increasing age, the transillumination defects of B6.Tyrp1b GpnmbR150X mice became more apparent as iris atrophy accompanied pigment dispersion. (G–I) As a consequence of an early-onset degenerative disease, the iris of B6-Lystbg-J mice appeared dark and granular. As observable in (I), cataracts were also common in B6-Lystbg-J eyes. (J–L) With transilluminating light, B6-Lystbg-J irides exhibited a distinct pattern of transillumination defects occurring in exfoliation syndrome characterized by concentric rings of transillumination.
Similar articles
- Elevated oxidative membrane damage associated with genetic modifiers of Lyst-mutant phenotypes.
Trantow CM, Hedberg-Buenz A, Iwashita S, Moore SA, Anderson MG. Trantow CM, et al. PLoS Genet. 2010 Jul 1;6(7):e1001008. doi: 10.1371/journal.pgen.1001008. PLoS Genet. 2010. PMID: 20617205 Free PMC article. - Lyst mutation in mice recapitulates iris defects of human exfoliation syndrome.
Trantow CM, Mao M, Petersen GE, Alward EM, Alward WL, Fingert JH, Anderson MG. Trantow CM, et al. Invest Ophthalmol Vis Sci. 2009 Mar;50(3):1205-14. doi: 10.1167/iovs.08-2791. Epub 2008 Nov 21. Invest Ophthalmol Vis Sci. 2009. PMID: 19029039 Free PMC article. - Genotype-phenotype associations in Danish patients with ocular and oculocutaneous albinism.
Kessel L, Kjer B, Lei U, Duno M, Grønskov K. Kessel L, et al. Ophthalmic Genet. 2021 Jun;42(3):230-238. doi: 10.1080/13816810.2021.1881979. Epub 2021 Feb 22. Ophthalmic Genet. 2021. PMID: 33612058 - Albinism and the associated ocular defects.
Oetting WS, Summers CG, King RA. Oetting WS, et al. Metab Pediatr Syst Ophthalmol (1985). 1994;17(1-4):5-9. Metab Pediatr Syst Ophthalmol (1985). 1994. PMID: 8719278 Review. - Tyrp1 and oculocutaneous albinism type 3.
Sarangarajan R, Boissy RE. Sarangarajan R, et al. Pigment Cell Res. 2001 Dec;14(6):437-44. doi: 10.1034/j.1600-0749.2001.140603.x. Pigment Cell Res. 2001. PMID: 11775055 Review.
Cited by
- Genetic modulation of the iris transillumination defect: a systems genetics analysis using the expanded family of BXD glaucoma strains.
Swaminathan S, Lu H, Williams RW, Lu L, Jablonski MM. Swaminathan S, et al. Pigment Cell Melanoma Res. 2013 Jul;26(4):487-98. doi: 10.1111/pcmr.12106. Epub 2013 May 13. Pigment Cell Melanoma Res. 2013. PMID: 23582180 Free PMC article. - Genetic modifiers as relevant biological variables of eye disorders.
Meyer KJ, Anderson MG. Meyer KJ, et al. Hum Mol Genet. 2017 Aug 1;26(R1):R58-R67. doi: 10.1093/hmg/ddx180. Hum Mol Genet. 2017. PMID: 28482014 Free PMC article. Review. - Genetic dissection of the Gpnmb network in the eye.
Lu H, Wang X, Pullen M, Guan H, Chen H, Sahu S, Zhang B, Chen H, Williams RW, Geisert EE, Lu L, Jablonski MM. Lu H, et al. Invest Ophthalmol Vis Sci. 2011 Jun 13;52(7):4132-42. doi: 10.1167/iovs.10-6493. Invest Ophthalmol Vis Sci. 2011. PMID: 21398278 Free PMC article. - Strain-specific differences in brain gene expression in a hydrocephalic mouse model with motile cilia dysfunction.
McKenzie CW, Preston CC, Finn R, Eyster KM, Faustino RS, Lee L. McKenzie CW, et al. Sci Rep. 2018 Sep 6;8(1):13370. doi: 10.1038/s41598-018-31743-5. Sci Rep. 2018. PMID: 30190587 Free PMC article. - Genomic insights into local adaptation and phenotypic diversity of Wenchang chickens.
Gu LH, Wu RR, Zheng XL, Fu A, Xing ZY, Chen YY, He ZC, Lu LZ, Qi YT, Chen AH, Zhang YP, Xu TS, Peng MS, Ma C. Gu LH, et al. Poult Sci. 2024 Mar;103(3):103376. doi: 10.1016/j.psj.2023.103376. Epub 2023 Dec 15. Poult Sci. 2024. PMID: 38228059 Free PMC article.
References
- Oetting WS, Fryer JP, Shriram S, King RA. Oculocutaneous albinism type 1: the last 100 years. Pigment Cell Res. 2003;16:307–311 - PubMed
- Ray K, Chaki M, Sengupta M. Tyrosinase and ocular diseases: some novel thoughts on the molecular basis of oculocutaneous albinism type 1. Prog Retin Eye Res. 2007;26:323–358 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 EY019485/EY/NEI NIH HHS/United States
- EY017673-02S2/EY/NEI NIH HHS/United States
- EY017673/EY/NEI NIH HHS/United States
- R01 EY017673/EY/NEI NIH HHS/United States
- EY019485/EY/NEI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases