NF-κB activity in muscle from obese and type 2 diabetic subjects under basal and exercise-stimulated conditions - PubMed (original) (raw)
NF-κB activity in muscle from obese and type 2 diabetic subjects under basal and exercise-stimulated conditions
Puntip Tantiwong et al. Am J Physiol Endocrinol Metab. 2010 Nov.
Abstract
NF-κB is a transcription factor that controls the gene expression of several proinflammatory proteins. Cell culture and animal studies have implicated increased NF-κB activity in the pathogenesis of insulin resistance and muscle atrophy. However, it is unclear whether insulin-resistant human subjects have abnormal NF-κB activity in muscle. The effect that exercise has on NF-κB activity/signaling also is not clear. We measured NF-κB DNA-binding activity and the mRNA level of putative NF-κB-regulated myokines interleukin (IL)-6 and monocyte chemotactic protein-1 (MCP-1) in muscle samples from T2DM, obese, and lean subjects immediately before, during (40 min), and after (210 min) a bout of moderate-intensity cycle exercise. At baseline, NF-κB activity was elevated 2.1- and 2.7-fold in obese nondiabetic and T2DM subjects, respectively. NF-κB activity was increased significantly at 210 min following exercise in lean (1.9-fold) and obese (2.6-fold) subjects, but NF-κB activity did not change in T2DM. Exercise increased MCP-1 mRNA levels significantly in the three groups, whereas IL-6 gene expression increased significantly only in lean and obese subjects. MCP-1 and IL-6 gene expression peaked at the 40-min exercise time point. We conclude that insulin-resistant subjects have increased basal NF-κB activity in muscle. Acute exercise stimulates NF-κB in muscle from nondiabetic subjects. In T2DM subjects, exercise had no effect on NF-κB activity, which could be explained by the already elevated NF-κB activity at baseline. Exercise-induced MCP-1 and IL-6 gene expression precedes increases in NF-κB activity, suggesting that other factors promote gene expression of these cytokines during exercise.
Figures
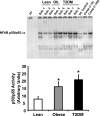
Fig. 1.
Basal NF-κB p50/p65 DNA-binding activity in skeletal muscle. NF-κB activity was measured in vastus lateralis muscle by EMSA. Graphic data are means ± SE. Representative blots are shown for 2–4 subjects (Sub). *P < 0.05 vs. lean group. An internal (Int) control (baboon muscle tissue) was utilized to normalize the data. To confirm the identity of the NF-κB p50/p65 band, increasing concentrations of a specific competitive (Comp) inhibitor were also tested (using sample from Sub. 8). Ob, obese; FP, free probe; T2DM, type 2 diabetes mellitus.
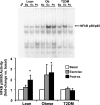
Fig. 2.
Effect of acute exercise on NF-κB p50/p65 activity in muscle. Biopsies were done at baseline (Ba), 40 min of exercise (Ex), and 210 min postexercise (Po), and NF-κB activity was measured by EMSA. Graphic data are means ± SE. Representative blots are shown for 1 subject/group. *P < 0.05 vs. basal of respective group. Data were normalized to the preexercise sample within each subject. FP, free probe.
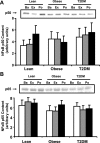
Fig. 3.
NF-κB p50 and p65 protein content in muscle. Biopsies were done at Ba, Ex, and Po, and NF-κB p50 (A) and p65 content (B) were measured by Western blotting. Graphic data are means ± SE. Representative blots are shown for 1 subject/group.
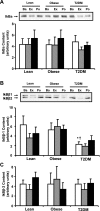
Fig. 4.
IκB protein content in muscle. Biopsies were done at Ba, Ex, and Po, and IκBα (A), IκBβ1 (B), and IκBβ2 content (C) were measured by Western blotting. Graphic data are means ± SE. Representative blots are shown for 1 subject/group; *P < 0.05 vs. lean at baseline; †P < 0.05 vs. obese at baseline.
Fig. 5.
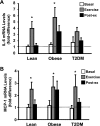
Effect of acute exercise on IL-6 (A) and monocyte chemotactic protein-1 (MCP-1; B) mRNA expression in muscle. Biopsies were done at baseline, 40 min of exercise, and 210 min postexercise, and IL-6 and MCP-1 mRNA expression were measured by real-time PCR. Data are means ± SE and normalized to lean at baseline. *P < 0.05 vs. basal of respective group.
Fig. 6.
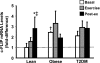
cellular-FLICE inhibitory protein (c-FLIP) mRNA levels. c-FLIP mRNA was measured before, during, and after exercise. Data are means ± SE and normalized to lean at baseline. *P < 0.05 vs. basal of lean group; ‡P < 0.05 vs. exercise of lean group; †P < 0.05 vs. lean at baseline.
Similar articles
- Elevated toll-like receptor 4 expression and signaling in muscle from insulin-resistant subjects.
Reyna SM, Ghosh S, Tantiwong P, Meka CS, Eagan P, Jenkinson CP, Cersosimo E, Defronzo RA, Coletta DK, Sriwijitkamol A, Musi N. Reyna SM, et al. Diabetes. 2008 Oct;57(10):2595-602. doi: 10.2337/db08-0038. Epub 2008 Jul 15. Diabetes. 2008. PMID: 18633101 Free PMC article. - Resistance exercise increases NF-κB activity in human skeletal muscle.
Vella L, Caldow MK, Larsen AE, Tassoni D, Della Gatta PA, Gran P, Russell AP, Cameron-Smith D. Vella L, et al. Am J Physiol Regul Integr Comp Physiol. 2012 Mar 15;302(6):R667-73. doi: 10.1152/ajpregu.00336.2011. Epub 2011 Dec 21. Am J Physiol Regul Integr Comp Physiol. 2012. PMID: 22189669 Clinical Trial. - Effect of a sustained reduction in plasma free fatty acid concentration on insulin signalling and inflammation in skeletal muscle from human subjects.
Liang H, Tantiwong P, Sriwijitkamol A, Shanmugasundaram K, Mohan S, Espinoza S, Defronzo RA, Dubé JJ, Musi N. Liang H, et al. J Physiol. 2013 Jun 1;591(11):2897-909. doi: 10.1113/jphysiol.2012.247510. Epub 2013 Mar 25. J Physiol. 2013. PMID: 23529132 Free PMC article. - Muscle atrophy in patients with Type 2 Diabetes Mellitus: roles of inflammatory pathways, physical activity and exercise.
Perry BD, Caldow MK, Brennan-Speranza TC, Sbaraglia M, Jerums G, Garnham A, Wong C, Levinger P, Asrar Ul Haq M, Hare DL, Price SR, Levinger I. Perry BD, et al. Exerc Immunol Rev. 2016;22:94-109. Exerc Immunol Rev. 2016. PMID: 26859514 Free PMC article. Review. - Reviewing physical exercise in non-obese diabetic Goto-Kakizaki rats.
Galán BSM, Serdan TDA, Rodrigues LE, Manoel R, Gorjão R, Masi LN, Pithon-Curi TC, Curi R, Hirabara SM. Galán BSM, et al. Braz J Med Biol Res. 2022 May 27;55:e11795. doi: 10.1590/1414-431X2022e11795. eCollection 2022. Braz J Med Biol Res. 2022. PMID: 35648976 Free PMC article. Review.
Cited by
- Exercise training modulates adipokine dysregulations in metabolic syndrome.
Babaei P, Hoseini R. Babaei P, et al. Sports Med Health Sci. 2022 Jan 20;4(1):18-28. doi: 10.1016/j.smhs.2022.01.001. eCollection 2022 Mar. Sports Med Health Sci. 2022. PMID: 35782776 Free PMC article. Review. - Muscle cells challenged with saturated fatty acids mount an autonomous inflammatory response that activates macrophages.
Pillon NJ, Arane K, Bilan PJ, Chiu TT, Klip A. Pillon NJ, et al. Cell Commun Signal. 2012 Oct 19;10(1):30. doi: 10.1186/1478-811X-10-30. Cell Commun Signal. 2012. PMID: 23078640 Free PMC article. - The Perfect Cup? Coffee-Derived Polyphenols and Their Roles in Mitigating Factors Affecting Type 2 Diabetes Pathogenesis.
Chapple B, Woodfin S, Moore W. Chapple B, et al. Molecules. 2024 Feb 6;29(4):751. doi: 10.3390/molecules29040751. Molecules. 2024. PMID: 38398503 Free PMC article. Review. - Unveiling the link between arsenic toxicity and diabetes: an in silico exploration into the role of transcription factors.
Fatema K, Haidar Z, Tanim MTH, Nath SD, Sajib AA. Fatema K, et al. Toxicol Res. 2024 Jul 18;40(4):653-672. doi: 10.1007/s43188-024-00255-y. eCollection 2024 Oct. Toxicol Res. 2024. PMID: 39345741 - Naringenin and Phytoestrogen 8-Prenylnaringenin Protect against Islet Dysfunction and Inhibit Apoptotic Signaling in Insulin-Deficient Diabetic Mice.
Park S, Sim KS, Hwangbo Y, Park SJ, Kim YJ, Kim JH. Park S, et al. Molecules. 2022 Jun 30;27(13):4227. doi: 10.3390/molecules27134227. Molecules. 2022. PMID: 35807469 Free PMC article.
References
- Al-Khalili L, Bouzakri K, Glund S, Lönnqvist F, Koistinen HA, Krook A. Signaling specificity of interleukin-6 action on glucose and lipid metabolism in skeletal muscle. Mol Endocrinol 20: 3364–3375, 2006 - PubMed
- Arkan MC, Hevener AL, Greten FR, Maeda S, Li ZW, Long JM, Wynshaw-Boris A, Poli G, Olefsky J, Karin M. IKK-beta links inflammation to obesity-induced insulin resistance. Nat Med 11: 191–198, 2005 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- CA-112175/CA/NCI NIH HHS/United States
- DK-80157/DK/NIDDK NIH HHS/United States
- AG-030979/AG/NIA NIH HHS/United States
- T32-HL-04776/HL/NHLBI NIH HHS/United States
- DK-24092/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous