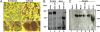Disruption of HDAC/CoREST/REST repressor by dnREST reduces genome silencing and increases virulence of herpes simplex virus - PubMed (original) (raw)
Disruption of HDAC/CoREST/REST repressor by dnREST reduces genome silencing and increases virulence of herpes simplex virus
Te Du et al. Proc Natl Acad Sci U S A. 2010.
Abstract
In nonneuronal cells, herpes simplex virus 1 overcomes host defenses, replicates, and ultimately kills the infected cell. Among the host defenses suppressed by the virus is a repressor complex whose key components are histone deacetylase (HDAC) 1 or 2, RE-1 silencing transcription factor (REST), corepressor of REST (CoREST), and lysine-specific demethylase (LSD) 1. In neurons innervating cells at the portal of entry into the body, the virus establishes a "latent" infection in which viral DNA is silenced with the exception of a family of genes. The question posed here is whether the virus hijacks this repressor complex to silence itself in neurons during the latent state. To test this hypothesis, we inserted into the wild-type virus genome a wild-type REST [recombinant (R) 111], a dominant-negative REST (dnREST) lacking the N- and C-terminal repressor domains (R112), or an insertion control consisting of tandem repeats of stop codons (R113). The recombinant virus R112 carrying the dnREST replicated better and was more virulent than the wild-type parent or the other recombinant viruses when administered by the corneal or i.p. routes. Moreover, in contrast to other recombinants, corneal route inoculation by R112 recombinant virus resulted in higher DNA copy numbers, higher levels of infectious virus in eye, trigeminal ganglion, or brain, and virtually complete destruction of trigeminal ganglia in mice that may ultimately succumb to infection. These results support an earlier conclusion that the HDAC/CoREST/REST/LSD1 repressor complex is a significant component of the host innate immunity and are consistent with the hypothesis that HSV-1 hijacks the repressor to silence itself during latent infection.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
Schematic representation of the functional domains of wild-type and dominant-negative REST and of the insertion control sequences inserted into the wild-type viral genome. Line 1: arrangement of REST drawn according to ref. . Line 2: DNA sequence arrangement of HSV-1 DNA. Line 3: schematic representation of wild-type REST flanked by the SV40 promoter and poly(A) sequence. Line 4: schematic representation of the dominant-negative REST shown lacking the repressor domains and N and C termini. Line 5: sequence arrangement of the insertion control sequence consisting of four stop codons, flanked by the SV40 promoter and poly(A) sequence. All constructs were inserted between UL3 and UL4 genes. NLS, nuclear localization signal.
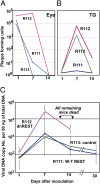
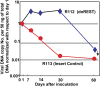
Fig. 2.
Quantification of infectious virus and viral DNA copies in the eye and trigeminal ganglia of mice inoculated with 105 pfu of virus per eye by the corneal route. (A and B) Eyes and TG were removed from euthanized mice, homogenized, and plated on Vero cell monolayers. The results shown are geometric mean titers. (C) The number of viral DNA copies per 50 ng of DNA was determined by quantitative PCR as described in Materials and Methods. The numbers shown are geometric mean titers based on assays of individual ganglia. Note that six mice inoculated with R112 recombinant virus succumbed to infection between days 12 and 14 after infection.
Fig. 3.
Quantification of viral DNA copies per 50 ng in TG of mice inoculated by the corneal route with 3 × 103 pfu of R112 or R113 recombinant virus. The assays were done on right ganglia harvested from six mice at each time point. For comparison, in this figure, the DNA copy numbers were normalized with respect to amounts detected on day 1 after virus inoculation on the cornea. The actual geometric mean averages of DNA copy number per 50 ng of DNA are shown in Table 1. Note that in this experiment, three of the mice inoculated with R112 succumbed to infection between days 9 and 14.
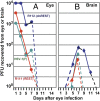
Fig. 4.
Quantification of infectious HSV-1 recovered from eyes and brains following inoculation by the corneal route of 105 pfu of wild-type parent virus [HSV-1(F)] or R111 or R112 recombinant viruses. The left eyes and brains were removed from euthanized mice, homogenized, and assayed on Vero cell monolayers. The results shown are geometric mean averages of virus recovered from six mice at each time point.
Fig. 5.
Expression of REST in TG of mice inoculated with the R112 recombinant virus. (A) TG were removed from uninfected mice or from mice on day 7 after infection with 105 pfu of R112 virus, fixed, sectioned, and stained with anti-REST antibody (Novus Biologicals) as detailed in Materials and Methods. Virtually identical images were observed with antibody to REST kindly provided by G. Mandel. (_A_1 and _A_2) Low- (100× optics) and high- (400× optics) magnification photographs of TG from uninfected mice. (_A_3 and _A_4) Corresponding low- and high-magnification photographs of TG harvested from mice 7 d after corneal inoculation. Red and black arrow points to REST in satellite cells and in dense intranuclear structures in neuronal cells, respectively. (B) At 7 d postinfection, 10 ganglia from uninfected or HSV-1(F)-infected mice were pooled, washed, and lysed in RIPA buffer. One hundred micrograms of total protein lysates was loaded onto 8% SDS/PAGE, transferred to PVDF membrane, and Western-blotted with anti-REST antibody (Novus Biologicals). (C) At 7 d postinfection, brain and spleen from uninfected mice and 10 ganglia from uninfected or HSV-1(F)-, R111-, and R112-infected mice were harvested and processed as described in B. One hundred micrograms of total protein lysates was loaded onto 7.5% SDS/PAGE, transferred to PVDF membrane, and Western-blotted with anti-REST antibody (Novus Biologicals).
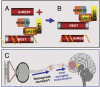
Fig. 6.
Schematic representation of the suppression of silencing of HSV DNA by dnREST. (A and B) Components of the repressor complex are assembled on the DNA and silence the DNA. dnREST bound to DNA precludes the binding of corepressors from binding to dnREST. In B, dnREST binds to DNA and precludes the assembly of the repressor complex. (C) Schematic representation of translocation of virus from the cornea to the brain. The virus is transported retrograde to the neuronal ganglion. Normally, the virus would establish latency and on reactivation be transported anterograde to the portal of entry (cornea). In the studies performed with the R112 recombinant virus, extensive replication and destruction of the ganglia could lead to postsynaptic transmission of the virus to the brain. In light of detection of virus in the brain of asymptomatic mice, it is conceivable that small amounts of virus “leak” into the brain on reactivation but that these do not cause symptomatic disease.
Similar articles
- HSV carrying WT REST establishes latency but reactivates only if the synthesis of REST is suppressed.
Zhou G, Du T, Roizman B. Zhou G, et al. Proc Natl Acad Sci U S A. 2013 Feb 5;110(6):E498-506. doi: 10.1073/pnas.1222497110. Epub 2013 Jan 22. Proc Natl Acad Sci U S A. 2013. PMID: 23341636 Free PMC article. - The checkpoints of viral gene expression in productive and latent infection: the role of the HDAC/CoREST/LSD1/REST repressor complex.
Roizman B. Roizman B. J Virol. 2011 Aug;85(15):7474-82. doi: 10.1128/JVI.00180-11. Epub 2011 Mar 30. J Virol. 2011. PMID: 21450817 Free PMC article. Review. - Engagement of the lysine-specific demethylase/HDAC1/CoREST/REST complex by herpes simplex virus 1.
Gu H, Roizman B. Gu H, et al. J Virol. 2009 May;83(9):4376-85. doi: 10.1128/JVI.02515-08. Epub 2009 Feb 4. J Virol. 2009. PMID: 19193804 Free PMC article. - The CoREST/REST repressor is both necessary and inimical for expression of herpes simplex virus genes.
Zhou G, Te D, Roizman B. Zhou G, et al. mBio. 2010 Dec 28;2(1):e00313-10. doi: 10.1128/mBio.00313-10. mBio. 2010. PMID: 21221247 Free PMC article. - Disturbed Yin-Yang balance: stress increases the susceptibility to primary and recurrent infections of herpes simplex virus type 1.
Yan C, Luo Z, Li W, Li X, Dallmann R, Kurihara H, Li YF, He RR. Yan C, et al. Acta Pharm Sin B. 2020 Mar;10(3):383-398. doi: 10.1016/j.apsb.2019.06.005. Epub 2019 Jun 22. Acta Pharm Sin B. 2020. PMID: 32140387 Free PMC article. Review.
Cited by
- Establishing a Herpesvirus Quiescent Infection in Differentiated Human Dorsal Root Ganglion Neuronal Cell Line Mediated by Micro-RNA Overexpression.
Chen YC, Li H, Martin-Caraballo M, Hsia SV. Chen YC, et al. Pathogens. 2022 Jul 16;11(7):803. doi: 10.3390/pathogens11070803. Pathogens. 2022. PMID: 35890047 Free PMC article. - A5-positive primary sensory neurons are nonpermissive for productive infection with herpes simplex virus 1 in vitro.
Bertke AS, Swanson SM, Chen J, Imai Y, Kinchington PR, Margolis TP. Bertke AS, et al. J Virol. 2011 Jul;85(13):6669-77. doi: 10.1128/JVI.00204-11. Epub 2011 Apr 20. J Virol. 2011. PMID: 21507969 Free PMC article. - Histone lysine-specific demethylase 1 (LSD1) protein is involved in Sal-like protein 4 (SALL4)-mediated transcriptional repression in hematopoietic stem cells.
Liu L, Souto J, Liao W, Jiang Y, Li Y, Nishinakamura R, Huang S, Rosengart T, Yang VW, Schuster M, Ma Y, Yang J. Liu L, et al. J Biol Chem. 2013 Nov 29;288(48):34719-28. doi: 10.1074/jbc.M113.506568. Epub 2013 Oct 25. J Biol Chem. 2013. PMID: 24163373 Free PMC article. - Checkpoints in productive and latent infections with herpes simplex virus 1: conceptualization of the issues.
Roizman B, Zhou G, Du T. Roizman B, et al. J Neurovirol. 2011 Dec;17(6):512-7. doi: 10.1007/s13365-011-0058-x. Epub 2011 Nov 4. J Neurovirol. 2011. PMID: 22052379 Review. - Epigenetics and Genetics of Viral Latency.
Lieberman PM. Lieberman PM. Cell Host Microbe. 2016 May 11;19(5):619-28. doi: 10.1016/j.chom.2016.04.008. Cell Host Microbe. 2016. PMID: 27173930 Free PMC article. Review.
References
- Roizman B, Knipe DM, Whitley RJ. Herpes simplex viruses. In: Knipe DM, et al., editors. Fields Virology. 5th Ed. New York: Lippincott Williams & Wilkins; 2007. pp. 2501–2601.
- Ballas N, Mandel G. The many faces of REST oversee epigenetic programming of neuronal genes. Curr Opin Neurobiol. 2005;15:500–506. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources