Novel application of Ki67 to quantify antigen-specific in vitro lymphoproliferation - PubMed (original) (raw)
. 2010 Oct 31;362(1-2):43-50.
doi: 10.1016/j.jim.2010.08.007. Epub 2010 Aug 25.
Lerisa Govender, Jane Hughes, Wendy Mavakla, Marwou de Kock, Charlene Barnard, Bernadette Pienaar, Esme Janse van Rensburg, Gail Jacobs, Gloria Khomba, Lynnette Stone, Brian Abel, Thomas J Scriba, Willem A Hanekom
Affiliations
- PMID: 20800066
- PMCID: PMC2989440
- DOI: 10.1016/j.jim.2010.08.007
Novel application of Ki67 to quantify antigen-specific in vitro lymphoproliferation
Andreia Soares et al. J Immunol Methods. 2010.
Abstract
Antigen-specific proliferation is a critical function of memory T cells that is often utilised to measure vaccine immunogenicity and T cell function. We proposed that measurement of intracellular expression of the nuclear protein, Ki67, could reliably assess specific T cell proliferation in vitro. Ki67 was expressed in CD4+ and CD8+ T cells that had undergone in vitro proliferation after 6-day culture of human whole blood or PBMC with antigens. T cells cultured with no antigen did not express Ki67. When compared to current flow cytometry based proliferation assays, Ki67 detected proliferating cells with greater sensitivity than BrdU incorporation, whereas its sensitivity was similar to dye dilution of Oregon Green (OG), a CFSE derivative. Overall, the magnitude and cytokine expression profile of proliferating T cells detected by Ki67 expression correlated strongly with T cells detected with BrdU or OG. The intra-assay variability of Ki67 proliferation was 2-3% for CD4+ T cells, and 10-16% for CD8+ T cells. Finally, we demonstrate that the Ki67 assay detects tetanus toxoid-specific CD4+ T cell proliferation after infant vaccination with tetanus toxoid (TT). Overall our data suggest that intracellular Ki67 expression provides a specific, quantitative and reproducible measure of antigen-specific T cell proliferation in vitro.
Copyright © 2010 Elsevier B.V. All rights reserved.
Figures
Fig. 1
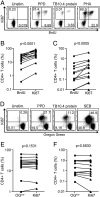
Ki67 as a specific marker of in vitro lymphoproliferation. Whole blood from healthy donors was incubated with the indicated antigens and Ki67 expression quantified on a daily basis over 6 days. (A) Representative example showing the frequencies of Ki67 expression by CD4+ T cells after incubation of whole blood with medium only (unstim.), PPD or αCD3/αCD28 over 6 days. Dotplots were gated on live, CD3+ CD4+ lymphocytes. Ki67+ CD4+ T cell frequencies after (B) PPD stimulation or (C) αCD3/αCD28 stimulation in 4 donors. Data are expressed as a percentage of the maximum response. The frequency of Ki67+ CD4+ T cells is indicated in each plot. (D) Frequencies of Ki67 expressing CD4+ T cells in whole blood from 15 donors after 6-day culture with medium only (unstim.) or PPD. (E) Frequencies of Ki67+ CD4+ T cells in PBMC from 14 donors. Differences were calculated using the Wilcoxon matched pairs test.
Fig. 2
Comparison of the Ki67 proliferation assay with the BrdU and Oregon Green proliferation assays. (A) Representative dotplots showing Ki67 versus BrdU expression by CD4+ T cells in whole blood. Dotplots are gated on live, CD3+ CD8− lymphocytes. Frequencies of (B) PPD- and (C) TB10.4-specific CD4+ T cell proliferation as detected by Ki67 expression or BrdU incorporation (n = 15). CD4+ T cells are defined as CD3+ CD8− T cells (see Data analysis). (D) Representative dotplots showing Ki67 and dye dilution of Oregon Green by CD4+ T cells in PBMC. Dotplots are gated on live, CD3+ CD8− lymphocytes. Frequencies of (E) PPD- and (F) TB10.4-specific CD4+ T cell proliferation as detected by Ki67 expression or dye dilution of Oregon Green (OGlow) in 14 donors. CD4+ T cells are defined as CD3+ CD8− T cells (see Data analysis). Differences were calculated using the Wilcoxon matched pairs test.
Fig. 3
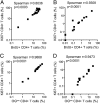
Correlations between Ki67+ CD4+ T cell expression and BrdU incorporation or dye dilution of Oregon Green (OGlow). Whole blood was incubated with (A) PPD or (C) TB10.4 for 6 days (n = 15). PBMC were incubated with (B) PPD or (D) TB10.4 for 6 days (n = 14). Correlations were calculated using a Spearman's rank correlation coefficient.
Fig. 4
Cytokine expression profiles of proliferating CD4+ T cells. Whole blood or PBMC were cultured for 6 days with no antigen or PPD. On day 6, cells were restimulated with PMA and ionomycin for 4 h in the presence of Brefeldin A to detect cytokine expression by proliferating T cells. Representative dotplots of the cytokine expression profiles of (A) Ki67+ or BrdU+ CD4+ T cells and (C) Ki67+ or OGlow CD4+ T cells. (B) Proportions of BrdU+, Ki67+ or Ki67+ BrdU− CD4+ T cells expressing IFN-γ, IL-2 or TNF-α (n = 15). (D) Proportions of Ki67+ or OGlow CD4+ T cells expressing IFN-γ, IL-2 or TNF-α (n = 14).
Fig. 5
Monitoring of vaccine-induced T cell proliferation. (A) Dotplots showing Ki67 expression by CD4+ T cells from a representative 18 month old toddler before (pre-TT) and after TT vaccination (post-TT). Dotplots are gated on live, CD3+ lymphocytes. Values in each dotplot represent the frequency of Ki67+ T cells within the CD3+ CD8− T cell population. Frequencies of (B) TT-specific and (C) BCG-specific CD4+ T cells pre- and post-TT vaccination in 11 toddlers. CD4+ T cells are defined as CD3+ CD8− T cells (see Data analysis). (D) Relative increase in TT-specific or BCG-specific CD4+ T cells pre- and post-TT. The lines represent the medians. (E) Dotplots depicting frequencies of Ki67+ CD4+ T cells in whole blood directly ex vivo or after culture in the absence of antigen (unstim.) for 6 days. Values in each dotplot represent the frequency of Ki67+ T cells within the CD3+ CD8− T cell population. (F) Frequencies of Ki67+ CD4+ T cells directly ex vivo or after culture for 6 days with medium (n = 11). CD4+ T cells are defined as CD3+ CD8− T cells (see Data analysis). Differences were calculated using the Wilcoxon matched pairs test.
Similar articles
- A minority of proliferating human CD4+ T cells in antigen-driven proliferation assays are antigen specific.
Bhattacharjee P, Pakusch M, Lacorcia M, Chiu CY, Liu X, Tresoldi E, Foster A, King L, Cameron FJ, Mannering SI. Bhattacharjee P, et al. Front Immunol. 2024 Oct 28;15:1491616. doi: 10.3389/fimmu.2024.1491616. eCollection 2024. Front Immunol. 2024. PMID: 39530093 Free PMC article. - CD4 T-helper cell cytokine phenotypes and antibody response following tetanus toxoid booster immunization.
Livingston KA, Jiang X, Stephensen CB. Livingston KA, et al. J Immunol Methods. 2013 Apr 30;390(1-2):18-29. doi: 10.1016/j.jim.2013.01.001. Epub 2013 Jan 11. J Immunol Methods. 2013. PMID: 23318779 - CD4+ cells proliferate after peanut-extract-specific and CD8+ cells proliferate after polyclonal stimulation of PBMC of children with atopic dermatitis.
Laan MP, Tibbe GJ, Oranje AP, Bosmans EP, Neijens HJ, Savelkoul HF. Laan MP, et al. Clin Exp Allergy. 1998 Jan;28(1):35-44. doi: 10.1046/j.1365-2222.1998.00168.x. Clin Exp Allergy. 1998. PMID: 9537777 - Antigen-induced death of mature T lymphocytes: analysis by flow cytometry.
Kabelitz D, Oberg HH, Pohl T, Pechhold K. Kabelitz D, et al. Immunol Rev. 1994 Dec;142:157-74. doi: 10.1111/j.1600-065x.1994.tb00888.x. Immunol Rev. 1994. PMID: 7698793 Review. No abstract available. - Memory cytolytic T-lymphocytes: induction, regulation and implications for vaccine design.
Baz A, Jackson DC, Kienzle N, Kelso A. Baz A, et al. Expert Rev Vaccines. 2005 Oct;4(5):711-23. doi: 10.1586/14760584.4.5.711. Expert Rev Vaccines. 2005. PMID: 16221072 Review.
Cited by
- HSP70 enhances immunosuppressive function of CD4(+)CD25(+)FoxP3(+) T regulatory cells and cytotoxicity in CD4(+)CD25(-) T cells.
Wachstein J, Tischer S, Figueiredo C, Limbourg A, Falk C, Immenschuh S, Blasczyk R, Eiz-Vesper B. Wachstein J, et al. PLoS One. 2012;7(12):e51747. doi: 10.1371/journal.pone.0051747. Epub 2012 Dec 26. PLoS One. 2012. PMID: 23300563 Free PMC article. - Regulatory T cells accumulate and proliferate in the ischemic hemisphere for up to 30 days after MCAO.
Stubbe T, Ebner F, Richter D, Engel O, Klehmet J, Royl G, Meisel A, Nitsch R, Meisel C, Brandt C. Stubbe T, et al. J Cereb Blood Flow Metab. 2013 Jan;33(1):37-47. doi: 10.1038/jcbfm.2012.128. Epub 2012 Sep 12. J Cereb Blood Flow Metab. 2013. PMID: 22968321 Free PMC article. - Adhesion analysis via a tumor vasculature-like microfluidic device identifies CD8+ T cells with enhanced tumor homing to improve cell therapy.
Camargo CP, Muhuri AK, Alapan Y, Sestito LF, Khosla M, Manspeaker MP, Smith AS, Paulos CM, Thomas SN. Camargo CP, et al. Cell Rep. 2023 Mar 28;42(3):112175. doi: 10.1016/j.celrep.2023.112175. Epub 2023 Feb 26. Cell Rep. 2023. PMID: 36848287 Free PMC article. - Derangement of cell cycle markers in peripheral blood mononuclear cells of asthmatic patients as a reliable biomarker for asthma control.
Hachim MY, Elemam NM, Ramakrishnan RK, Salameh L, Olivenstein R, Hachim IY, Venkatachalam T, Mahboub B, Al Heialy S, Hamid Q, Hamoudi R. Hachim MY, et al. Sci Rep. 2021 Jun 4;11(1):11873. doi: 10.1038/s41598-021-91087-5. Sci Rep. 2021. PMID: 34088958 Free PMC article. - Methods to Assess Proliferation of Stimulated Human Lymphocytes In Vitro: A Narrative Review.
Ganesan N, Ronsmans S, Hoet P. Ganesan N, et al. Cells. 2023 Jan 20;12(3):386. doi: 10.3390/cells12030386. Cells. 2023. PMID: 36766728 Free PMC article. Review.
References
- Bachmann M.F., Wolint P. Functional properties and lineage relationship of CD8+ T cell subsets identified by expression of IL-7 receptor alpha and CD62L. J. Immunol. 2005;175(7):4686. - PubMed
- Cellerai C., Harari A. Functional and phenotypic characterization of tetanus toxoid-specific human CD4+ T cells following re-immunization. Eur. J. Immunol. 2007;37(4):1129. - PubMed
- Doisne J.M., Urrutia A. CD8+ T cells specific for EBV, cytomegalovirus, and influenza virus are activated during primary HIV infection. J. Immunol. 2004;173(4):2410. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AI70022/AI/NIAID NIH HHS/United States
- N01 AI070022/AI/NIAID NIH HHS/United States
- 080929/WT_/Wellcome Trust/United Kingdom
- 080929/Z/06/Z/WT_/Wellcome Trust/United Kingdom
- R01-AI065653/AI/NIAID NIH HHS/United States
- R01 AI065653/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous