Molecular therapy targeting Sonic hedgehog and hepatocyte growth factor signaling in a mouse model of medulloblastoma - PubMed (original) (raw)
Molecular therapy targeting Sonic hedgehog and hepatocyte growth factor signaling in a mouse model of medulloblastoma
Valerie Coon et al. Mol Cancer Ther. 2010 Sep.
Abstract
The use of genetically engineered mice has provided insights into the molecular pathogenesis of the pediatric brain tumor medulloblastoma and revealed promising therapeutic targets. Ectopic expression of Sonic hedgehog (Shh) in cerebellar neural progenitor cells induces medulloblastomas in mice, and coexpression of hepatocyte growth factor (HGF) enhances Shh-induced tumor formation. To determine whether Shh + HGF-driven medulloblastomas were responsive to Shh signaling blockade and whether treatment response could be enhanced by combination therapy targeting both HGF and Shh signaling pathways, we carried out a survival study in mice. We induced medulloblastomas by retrovirus-mediated expression of Shh and HGF, after which we treated the mice systemically with (a) HGF-neutralizing monoclonal antibody L2G7, (b) Shh signaling inhibitor cyclopamine, (c) Shh-neutralizing monoclonal antibody 5E1, (d) L2G7 + cyclopamine, or (e) L2G7 + 5E1. We report that monotherapy targeting either HGF signaling or Shh signaling prolonged survival and that anti-HGF therapy had a more durable response than Shh-targeted therapy. The effect of L2G7 + 5E1 combination therapy on cumulative survival was equivalent to that of L2G7 monotherapy and that of L2G7 + cyclopamine therapy was worse. The principal mechanism by which Shh- and HGF-targeted therapies inhibited tumor growth was a potent apoptotic death response in tumor cells, supplemented by a weaker suppressive effect on proliferation. Our observation that combination therapy either failed to improve or even reduced survival in mice bearing Shh + HGF-induced medulloblastomas compared with monotherapy underscores the importance of preclinical testing of molecular-targeted therapies in animal models of tumors in which the targeted pathways are known to be active.
Figures
Figure 1
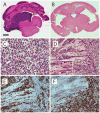
Histopathology of medulloblastomas induced by Shh+HGF. A–B, coronal brain sections showing tumor in the cerebellum and fourth ventricle (A) and obstructive hydrocephalus in the forebrain (B) (H&E). C, classic medulloblastoma pattern showing homogeneous sheets of undifferentiated tumor cells (H&E). D, MBEN pattern, in which round, neurocytic-appearing tumor cells are arrayed in a linear pattern (upper left and inset), adjacent to an area of disorganized cytoarchitecture characteristic of the classic pattern (right) (H&E). E, immunoperoxidase staining showing that the MBEN areas (left) show abundant immunoreactivity for neuronal differentiation marker NeuN compared with classic-appearing areas (right). F, immunoperoxidase staining showing that Ki67 staining is absent in the MBEN area (left) compared with the actively proliferating cells in the classic-appearing area (right). Scale bar, 500 μm (A–B), 25 μm (C and D inset), 50 μm (D–F).
Figure 2
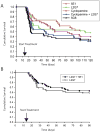
Response to Shh- and HGF-targeted therapy in mice with medulloblastomas. Kaplan-Meier survival analysis of mice injected with RCAS-Shh and RCAS-HGF on day 0 and then treated with the indicated therapeutic agents starting on day 14 (arrow). A and B show results from two independent experiments.
Figure 3
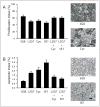
Analysis of proliferation and apoptosis in Shh+HGF–induced medulloblastomas. Bar graphs and representative photomicrographs showing the percentage (mean ± SEM) of tumor cells with positive immunoreactive staining for Ki67 (proliferation index) (A) and cleaved caspase-3 (apoptotic index) (B) in Shh+HGF–induced medulloblastomas from mice treated with the indicated therapeutic agents. Scale bar, 25 μm.
Figure 4
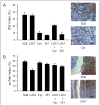
Analysis of Shh and HGF signaling in response to molecular-targeted therapy. Bar graphs and representative photomicrographs showing the percentage (mean ± SEM) of tumor cells with positive immunoreactive staining for Gli2 in the nucleus (Gli2 index) (A) and phosphorylated c-Met in the cytoplasm (pc-Met index) (B) in medulloblastomas from mice treated with the indicated therapeutic agents. Phototmicrographs in (B) show pc-Met+ tumor cells (right) adjacent to cerebellar cortex (left). Scale bar, 25 μm (A) and 50 μm (B).
Similar articles
- Hepatocyte growth factor and sonic Hedgehog expression in cerebellar neural progenitor cells costimulate medulloblastoma initiation and growth.
Binning MJ, Niazi T, Pedone CA, Lal B, Eberhart CG, Kim KJ, Laterra J, Fults DW. Binning MJ, et al. Cancer Res. 2008 Oct 1;68(19):7838-45. doi: 10.1158/0008-5472.CAN-08-1899. Cancer Res. 2008. PMID: 18829539 Free PMC article. - Apoptosis suppression by somatic cell transfer of Bcl-2 promotes Sonic hedgehog-dependent medulloblastoma formation in mice.
McCall TD, Pedone CA, Fults DW. McCall TD, et al. Cancer Res. 2007 Jun 1;67(11):5179-85. doi: 10.1158/0008-5472.CAN-06-4177. Cancer Res. 2007. PMID: 17545597 - The miR-17/92 polycistron is up-regulated in sonic hedgehog-driven medulloblastomas and induced by N-myc in sonic hedgehog-treated cerebellar neural precursors.
Northcott PA, Fernandez-L A, Hagan JP, Ellison DW, Grajkowska W, Gillespie Y, Grundy R, Van Meter T, Rutka JT, Croce CM, Kenney AM, Taylor MD. Northcott PA, et al. Cancer Res. 2009 Apr 15;69(8):3249-55. doi: 10.1158/0008-5472.CAN-08-4710. Epub 2009 Apr 7. Cancer Res. 2009. PMID: 19351822 Free PMC article. - Modeling medulloblastoma with genetically engineered mice.
Fults DW. Fults DW. Neurosurg Focus. 2005 Nov 15;19(5):E7. doi: 10.3171/foc.2005.19.5.8. Neurosurg Focus. 2005. PMID: 16398471 Review. - Pseudo-active sites of protease domains: HGF/Met and Sonic hedgehog signaling in cancer.
Maun HR, Kirchhofer D, Lazarus RA. Maun HR, et al. Biol Chem. 2010 Aug;391(8):881-92. doi: 10.1515/BC.2010.098. Biol Chem. 2010. PMID: 20536384 Review.
Cited by
- The role of Hedgehog and Notch signaling pathway in cancer.
Xia R, Xu M, Yang J, Ma X. Xia R, et al. Mol Biomed. 2022 Dec 15;3(1):44. doi: 10.1186/s43556-022-00099-8. Mol Biomed. 2022. PMID: 36517618 Free PMC article. Review. - Matching mice to malignancy: molecular subgroups and models of medulloblastoma.
Lau J, Schmidt C, Markant SL, Taylor MD, Wechsler-Reya RJ, Weiss WA. Lau J, et al. Childs Nerv Syst. 2012 Apr;28(4):521-32. doi: 10.1007/s00381-012-1704-1. Childs Nerv Syst. 2012. PMID: 22315164 Free PMC article. Review. - MiR-338* targeting smoothened to inhibit pulmonary fibrosis by epithelial-mesenchymal transition.
Zhuang Y, Dai J, Wang Y, Zhang H, Li X, Wang C, Cao M, Liu Y, Ding J, Cai H, Zhang D, Wang Y. Zhuang Y, et al. Am J Transl Res. 2016 Jul 15;8(7):3206-13. eCollection 2016. Am J Transl Res. 2016. PMID: 27508042 Free PMC article. - Development of anticancer agents targeting the Hedgehog signaling.
Zhang X, Tian Y, Yang Y, Hao J. Zhang X, et al. Cell Mol Life Sci. 2017 Aug;74(15):2773-2782. doi: 10.1007/s00018-017-2497-x. Epub 2017 Mar 17. Cell Mol Life Sci. 2017. PMID: 28314894 Free PMC article. Review. - miRNA-338-3p suppresses cell growth of human colorectal carcinoma by targeting smoothened.
Sun K, Deng HJ, Lei ST, Dong JQ, Li GX. Sun K, et al. World J Gastroenterol. 2013;19(14):2197-207. doi: 10.3748/wjg.v19.i14.2197. World J Gastroenterol. 2013. PMID: 23599646 Free PMC article.
References
- Fomchenko EI, Holland EC. Mouse models of brain tumors and their applications in preclinical trials. Clin Cancer Res. 2006;12:5288–97. - PubMed
- Crawford JR, MacDonald TJ, Packer RJ. Medulloblastoma in childhood: new biological advances. Lancet Neurol. 2007;6:1073–85. - PubMed
- Gajjar A, Chintagumpala M, Ashley D, et al. Risk-adapted craniospinal radiotherapy followed by high-dose chemotherapy and stem-cell rescue in children with newly diagnosed medulloblastoma (St Jude Medulloblastoma-96): long-term results from a prospective, multicentre trial. Lancet Oncol. 2006;7:813–20. - PubMed
- Packer RJ, Sutton LN, Atkins TE, et al. A prospective study of cognitive function in children receiving whole-brain radiotherapy and chemotherapy: 2-year results. J Neurosurg. 1989;70:707–13. - PubMed
- Silber JH, Littman PS, Meadows AT. Stature loss following skeletal irradiation for childhood cancer. J Clin Oncol. 1990;8:304–12. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- CA129192/CA/NCI NIH HHS/United States
- R01 CA108622/CA/NCI NIH HHS/United States
- NS43987/NS/NINDS NIH HHS/United States
- R01 CA108622-05/CA/NCI NIH HHS/United States
- R01 CA129192/CA/NCI NIH HHS/United States
- CA108622/CA/NCI NIH HHS/United States
- R01 NS043987/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical