A high-fat, ketogenic diet causes hepatic insulin resistance in mice, despite increasing energy expenditure and preventing weight gain - PubMed (original) (raw)
A high-fat, ketogenic diet causes hepatic insulin resistance in mice, despite increasing energy expenditure and preventing weight gain
François R Jornayvaz et al. Am J Physiol Endocrinol Metab. 2010 Nov.
Abstract
Low-carbohydrate, high-fat ketogenic diets (KD) have been suggested to be more effective in promoting weight loss than conventional caloric restriction, whereas their effect on hepatic glucose and lipid metabolism and the mechanisms by which they may promote weight loss remain controversial. The aim of this study was to explore the role of KD on liver and muscle insulin sensitivity, hepatic lipid metabolism, energy expenditure, and food intake. Using hyperinsulinemic-euglycemic clamps, we studied insulin action in mice fed a KD or regular chow (RC). Body composition was assessed by ¹H magnetic resonance spectroscopy. Despite being 15% lighter (P < 0.001) than RC-fed mice because of a 17% increase in energy expenditure (P < 0.001), KD-fed mice manifested severe hepatic insulin resistance, as reflected by decreased suppression (0% vs. 100% in RC-fed mice, P < 0.01) of endogenous glucose production during the clamp. Hepatic insulin resistance could be attributed to a 350% increase in hepatic diacylglycerol content (P < 0.001), resulting in increased activation of PKCε (P < 0.05) and decreased insulin receptor substrate-2 tyrosine phosphorylation (P < 0.01). Food intake was 56% (P < 0.001) lower in KD-fed mice, despite similar caloric intake, and could partly be attributed to a more than threefold increase (P < 0.05) in plasma N-acylphosphatidylethanolamine concentrations. In conclusion, despite preventing weight gain in mice, KD induces hepatic insulin resistance secondary to increased hepatic diacylglycerol content. Given the key role of nonalcoholic fatty liver disease in the development of type 2 diabetes and the widespread use of KD for the treatment of obesity, these results may have potentially important clinical implications.
Figures
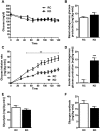
Fig. 1.
Ketogenic diet (KD) causes hepatic insulin resistance in mice. A: euglycemia (100–120 mg/dl) was maintained throughout the clamp in mice fed KD and those fed regular chow (RC). B: basal endogenous glucose production was similar between groups. C: glucose infusion rates were significantly lower in KD-fed mice. D: ability of insulin to suppress clamp endogenous glucose production was significantly impaired in KD-fed mice. E and F: glycolysis and glycogen synthesis were similar between groups. Values are means ± SE (n = 9 per group). **P < 0.01; ***P < 0.001 vs. RC.
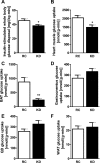
Fig. 2.
KD causes a decrease in insulin-stimulated whole body glucose disposal. A: insulin-stimulated whole body glucose disposal was significantly lower in KD-fed mice (n = 9 per group). This lower level of insulin-stimulated whole body glucose disposal was due to decreased heart muscle glucose uptake (B), as well as decreased brown adipose tissue (BAT) glucose uptake (C); there was no difference in muscle [gastrocnemius (D) and quadriceps (QD, E)] or white adipose tissue (WAT, F) glucose uptake (n = 8 per group). Values are means ± SE. *P < 0.05; **P < 0.01 vs. RC.
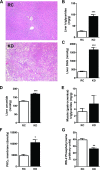
Fig. 3.
KD increases liver lipid metabolites and impairs hepatic insulin signaling. A: histological evidence of nonalcoholic fatty liver disease in KD-fed mice with microvesicular pattern lipid infiltration (hematoxylin and eosin staining, ×100 magnification). B–D: increase in liver lipid metabolites. Triglycerides (B), diacylglycerol (DAG, C), and ceramide (D) were significantly increased in KD-fed mice (n = 6 per group). Consequently, in KD-fed mice, PKCε was significantly translocated to the membrane (F), and insulin receptor substrate-2 (IRS-2) tyrosine phosphorylation was decreased (G) (n = 4–5 per group). Muscle (gastrocnemius) triglyceride content (E) was not different between groups. Values are means ± SE (n = 6 per group). *P < 0.05; **P < 0.01; ***P < 0.001 vs. RC.
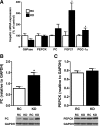
Fig. 4.
KD modifies gene expression regulating hepatic glucose metabolism. A: liver mRNA expression was not different for glucose-6-phosphatase (G6Pase), phospho_enol_pyruvate carboxykinase (PEPCK), and pyruvate carboxylase (PC), whereas fibroblast growth factor-21 (FGF21) and peroxisome proliferator-activated receptor-γ coactivator-1α (PGC-1α) mRNA were significantly increased (n = 6 per group). PC protein level (B) was significantly increased in KD-fed mice, whereas PEPCK protein level (C) was not different (n = 4–5 per group). Values are means ± SE. *P < 0.05 vs. RC.
Similar articles
- Thyroid hormone receptor-α gene knockout mice are protected from diet-induced hepatic insulin resistance.
Jornayvaz FR, Lee HY, Jurczak MJ, Alves TC, Guebre-Egziabher F, Guigni BA, Zhang D, Samuel VT, Silva JE, Shulman GI. Jornayvaz FR, et al. Endocrinology. 2012 Feb;153(2):583-91. doi: 10.1210/en.2011-1793. Epub 2011 Dec 6. Endocrinology. 2012. PMID: 22147010 Free PMC article. - Short-term feeding of a ketogenic diet induces more severe hepatic insulin resistance than an obesogenic high-fat diet.
Grandl G, Straub L, Rudigier C, Arnold M, Wueest S, Konrad D, Wolfrum C. Grandl G, et al. J Physiol. 2018 Oct;596(19):4597-4609. doi: 10.1113/JP275173. Epub 2018 Aug 8. J Physiol. 2018. PMID: 30089335 Free PMC article. - Hepatic steatosis, inflammation, and ER stress in mice maintained long term on a very low-carbohydrate ketogenic diet.
Garbow JR, Doherty JM, Schugar RC, Travers S, Weber ML, Wentz AE, Ezenwajiaku N, Cotter DG, Brunt EM, Crawford PA. Garbow JR, et al. Am J Physiol Gastrointest Liver Physiol. 2011 Jun;300(6):G956-67. doi: 10.1152/ajpgi.00539.2010. Epub 2011 Mar 31. Am J Physiol Gastrointest Liver Physiol. 2011. PMID: 21454445 Free PMC article. - Ketogenic Diet and Weight Loss: Is There an Effect on Energy Expenditure?
Basolo A, Magno S, Santini F, Ceccarini G. Basolo A, et al. Nutrients. 2022 Apr 26;14(9):1814. doi: 10.3390/nu14091814. Nutrients. 2022. PMID: 35565778 Free PMC article. Review. - Nav1.8 neurons are involved in limiting acute phase responses to dietary fat.
Udit S, Burton M, Rutkowski JM, Lee S, Bookout AL, Scherer PE, Elmquist JK, Gautron L. Udit S, et al. Mol Metab. 2017 Oct;6(10):1081-1091. doi: 10.1016/j.molmet.2017.07.012. Epub 2017 Aug 4. Mol Metab. 2017. PMID: 29031710 Free PMC article. Review.
Cited by
- Adding more fat to a high-fat diet only exacerbates hepatic insulin resistance.
Medak KD, Townsend LK. Medak KD, et al. J Physiol. 2019 Mar;597(6):1435-1436. doi: 10.1113/JP277632. Epub 2019 Jan 31. J Physiol. 2019. PMID: 30653266 Free PMC article. No abstract available. - Very low-carbohydrate, high-fat, weight reduction diet decreases hepatic gene response to glucose in obese rats.
Axen KV, Harper MA, Kuo YF, Axen K. Axen KV, et al. Nutr Metab (Lond). 2018 Jul 31;15:54. doi: 10.1186/s12986-018-0284-9. eCollection 2018. Nutr Metab (Lond). 2018. PMID: 31061673 Free PMC article. - Diets, Gut Microbiota and Metabolites.
Liu Y, Zhong W, Li X, Shen F, Ma X, Yang Q, Hong S, Sun Y. Liu Y, et al. Phenomics. 2023 Mar 2;3(3):268-284. doi: 10.1007/s43657-023-00095-0. eCollection 2023 Jun. Phenomics. 2023. PMID: 37325710 Free PMC article. Review.
References
- Atkins RC. Dr. Atkins' New Diet Revolution. New York: Quill, 2002.
- Badman MK, Pissios P, Kennedy AR, Koukos G, Flier JS, Maratos-Flier E. Hepatic fibroblast growth factor 21 is regulated by PPARα and is a key mediator of hepatic lipid metabolism in ketotic states. Cell Metab 5: 426–437, 2007. - PubMed
- Bisschop PH, de Metz J, Ackermans MT, Endert E, Pijl H, Kuipers F, Meijer AJ, Sauerwein HP, Romijn JA. Dietary fat content alters insulin-mediated glucose metabolism in healthy men. Am J Clin Nutr 73: 554–559, 2001. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 DK-40936/DK/NIDDK NIH HHS/United States
- U24 DK059635/DK/NIDDK NIH HHS/United States
- R01 DK040936/DK/NIDDK NIH HHS/United States
- P30 DK045735/DK/NIDDK NIH HHS/United States
- U24 DK-076169/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical