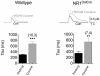NMDA receptors on non-dopaminergic neurons in the VTA support cocaine sensitization - PubMed (original) (raw)
. 2010 Aug 16;5(8):e12141.
doi: 10.1371/journal.pone.0012141.
Cameron H Good, Oscar Diaz-Ruiz, YaJun Zhang, Alexander F Hoffman, Lufei Shan, Serena Y Kuang, Nasir Malik, Vladimir I Chefer, Andreas C Tomac, Carl R Lupica, Cristina M Bäckman
Affiliations
- PMID: 20808436
- PMCID: PMC2922329
- DOI: 10.1371/journal.pone.0012141
NMDA receptors on non-dopaminergic neurons in the VTA support cocaine sensitization
Yu Luo et al. PLoS One. 2010.
Abstract
Background: The initiation of behavioral sensitization to cocaine and other psychomotor stimulants is thought to reflect N-methyl-D-aspartate receptor (NMDAR)-mediated synaptic plasticity in the mesolimbic dopamine (DA) circuitry. The importance of drug induced NMDAR mediated adaptations in ventral tegmental area (VTA) DA neurons, and its association with drug seeking behaviors, has recently been evaluated in Cre-loxp mice lacking functional NMDARs in DA neurons expressing Cre recombinase under the control of the endogenous dopamine transporter gene (NR1(DATCre) mice).
Methodology and principal findings: Using an additional NR1(DATCre) mouse transgenic model, we demonstrate that while the selective inactivation of NMDARs in DA neurons eliminates the induction of molecular changes leading to synaptic strengthening, behavioral measures such as cocaine induced locomotor sensitization and conditioned place preference remain intact in NR1(DATCre) mice. Since VTA DA neurons projecting to the prefrontal cortex and amygdala express little or no detectable levels of the dopamine transporter, it has been speculated that NMDA receptors in DA neurons projecting to these brain areas may have been spared in NR1(DATCre) mice. Here we demonstrate that the NMDA receptor gene is ablated in the majority of VTA DA neurons, including those exhibiting undetectable DAT expression levels in our NR1(DATCre) transgenic model, and that application of an NMDAR antagonist within the VTA of NR1(DATCre) animals still blocks sensitization to cocaine.
Conclusions/significance: These results eliminate the possibility of NMDAR mediated neuroplasticity in the different DA neuronal subpopulations in our NR1(DATCre) mouse model and therefore suggest that NMDARs on non-DA neurons within the VTA must play a major role in cocaine-related addictive behavior.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
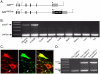
Figure 1. Cre mediated recombination of the NMDAR gene occurs in most ventral tegmental DA neurons with reduced DAT expression levels.
(A) Maps showing the endogenous DAT gene (DATwt), and targeted variant DATCre. Black unlabeled boxes represent translated exons of the DAT gene and the light grey box shows the 3′UTR. The stop codon has been labeled with an asterisk. A targeting vector containing the Cre recombinase gene (nlsCre) preceded by an IRES (i) was targeted by homologous recombination into the 3′UTR of the DAT gene. (B) PCR screen for Cre-mediated Grin1 exon 11–22 deletion. Genomic DNAs were prepared from the indicated tissues from a NR1DATCre mouse. PCR analyses show Grin1 deletion (Δ_loxP_) is specific to the olfactory bulb and VTA/SN. Grin1 deletion was not detected in other brain areas or tissues analyzed. (C) Confocal image of DAT-(green) and TH-(red) immunoreactive neurons located in the parabrachial nucleus of the VTA in a NR1DATCre animal. TH immunoreactive neuron pointed with a white arrow does not show detectable DAT immunoreactivity. TH immunoreactive neurons (2–5 neurons per sample) with undetectable (small arrow, n = 36 samples) and detectable DAT levels (n = 15 samples) were collected by laser-microdissection for DNA extraction and PCR processing. Scale bar = 30 µm. (D) PCR analyses of laser-microdissected neurons from NR1DATCre animals revealed Cre mediated recombination in DA neurons with both, low and high DAT protein levels. DNA extracted from tissue surrounding DA neurons within the VTA did not recombine at the NMDAR gene locus.
Figure 2. Absence of functional NMDAR in NR1DATCre mice.
(A) Example waveforms recorded at +50 mV from both wildtype (WT) and NR1DATCre (KO) cells before (black trace) and after AP5 application (grey trace). In WT cells, the NMDA component could be obtained by subtracting the AMPA current remaining after AP5 application from the control current, as indicated. In KO cells, only the AMPA mediated EPSC was observed. The gray block represents the time over which the NMDA current was measured. Scale bars are 20 pA and 20 ms. (B) Average NMDA current measured as described in A. Responses were evoked at holding potentials, beginning at −90 mV in 10 mV increments to +50mV. (C) Decay time constant (tau) of EPSCs measured before and after AP5 in control and KO cells. Following AP5 superfusion, the remaining current in the WT cells exhibited similar decay constants as the KO animals. (D) Mean time course of NMDA currents before and after AP5 application. Following AP5 the WT response amplitude was reduced to the same level observed in the KO cells, thus confirming the lack of active NMDA channels in DA NR1DATCre neurons.
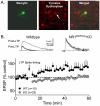
Figure 3. LTP evoked by high frequency afferent stimulation is not observed in DA neurons from NR1DATCre mice.
(A) Photomicrograph of a recorded VTA neuron. The left panel shows a biocytin labeled neuron, the middle panel shows TH immunohistochemistry in the same brain slice, and the right panel shows a merged image demonstrating the overlap of biocytin and TH staining. Scale bar = 40 µm. (B) Mean time course of EPSPs before and after LTP initiation (arrow) in both WT and NR1-KO cells. Scale bars are 5 mV and 25 ms. The spike timing protocol did not lead to LTP in any of the KO cells tested (n = 23, gray circles). However, all 10 WT DA cells demonstrated LTP (black circles).
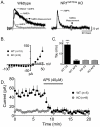
Figure 4. Summary of DA release in the NAc following deletion of NR1 in DA neurons.
DA release was elicited by local, single pulse (1 ms) electrical stimulation in brain slices, and the DA signal was monitored using fast scan cyclic voltammetry (FSCV). (A) Input-output relationship (current intensity versus peak DA signal) from slices obtained from WT (black circles) and NR1DATCre (grey circles) mice. Number of slices and number of mice are noted in parentheses. A significantly greater release was observed in NR1DATCre mice (Two way repeated measures ANOVA; genotype x stimulus intensity, F440,10 = 1.91, p = 0.04). Acute blockade of NMDA receptors does not alter DA release in the NAc. (B) Representative DA signals elicited by single pulse stimulation in a WT mouse prior to (control) and 15 minutes following AP5 application (40 µM). Inset of the control response shows the cyclic voltammogram obtained during the peak of the signal, confirming the identity of the DA signal. Summary of AP5 effects on DA signals elicited by single pulse stimulation (n = 10). Signals were obtained every 2 min. AP5 was applied during the time indicated by the gray bar. (C) Summary of frequency-response curves obtained in WT (black circles) and NR1DATCre mice (grey circles). A significant reduction in DA release was observed in the NR1DATCre mice (two way RM-ANOVA; genotype x frequency, F160,4 = 3.57, p = 0.008). Summary of frequency-response curves obtained in the absence (control, filled circles) and presence (AP5, open circles) of AP5 (n = 10). No significant effect of NMDA antagonism was seen (two way RM-ANOVA; treatment x frequency, F72, 4 = 1.46, p = 0.2239).
Figure 5. Acute effects of cocaine in NAc slices.
Voltammetric traces of DA signals taken prior to (black) and following bath application of 500 nM cocaine (gray). Cocaine significantly increased both the amplitude and the decay time constant (tau) in slices from both WT (10 slices from 3 subjects) and NR1DATCre (7 slices from 4 subjects) mice. **p<0.001; ***p<0.0001, paired t-test.
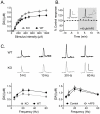
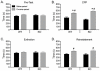
Figure 6. Basal locomotor activity in NR1DATCre mice.
(A) Locomotor response to novel environment. Total distance traveled in the first hour in the locomotor chamber in WT (n = 8) and NR1DATCre KO (n = 8) animals. KO animals were significantly less active during the first hour presented to the activity chambers, p<0.05, two-tailed t-test. (B) Total distance traveled in 24 hours in the locomotor chamber. There were not significant differences between the genotypes, p = 0.519, two-tailed t-test. (C) Day-night locomotion in a 24 hr circadian cycle at 1 hr intervals in WT and NR1DATCre animals.
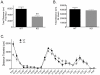
Figure 7. Cocaine induced sensitization in NR1DATCre mice.
(A) Context dependent sensitization. Cumulative 30 min locomotor response to cocaine (15 mg/kg, i.p.) in WT (n = 7) and NR1DATCre (n = 8) animals at different days after injection. S2, day 2 of saline injection. C4, day 4 of cocaine injection. C20 represents a challenge cocaine injection after withdrawal. Cocaine exposure in association with a new environment induced significant sensitization in both WT and NR1DATCre mice (two way ANOVA, Newman-Keuls post hoc for all genotypes: *p<0.05 for WT compared to saline injection, ** p<0.01 for KO compared to saline injection; #p<0.05 for WT compared to C1, ## p<0.01 for KO compared to C1). No statistical differences were observed between genotypes neither at the initial development of sensitization (C1 versus C4) nor at the challenge injection after withdrawal (C20, two way RM-ANOVA, genotypes, F1, 65 = 0.00703, p = 0.934). (B) Cocaine-induced sensitization in the home cage. Cumulative 30 min locomotor response to cocaine in WT and NR1DATCre animals pretreated with saline (WT, n = 8 and KO, n = 6) or 20 mg/kg cocaine, i.p. (WT, n = 8 and KO, n = 7) in the home cage, prior to cocaine challenge (15 mg/kg, i.p.) in activity chambers. Cocaine exposure in the home cage induced significant sensitization in both, WT and NR1DATCre mice (Two way ANOVA, F1,28 = 3.57, p<0.001; Newman-Keuls post hoc for all genotypes: * p<0.05 for KO, ** p<0.01 for WT). Data presented as mean ± SEM.
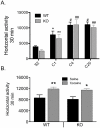
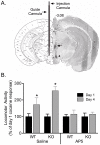
Figure 8. Effect of pretreatment with AP5 in the VTA on the development of behavioral sensitization to cocaine in NR1DATCre mice.
(A) Location of the guide cannula and the injection cannula in the ventral tegmental area (VTA). Left panel is the illustration from the mouse brain atlas , indicating millimeters from bregma. The right half shows the image with dyes indicating the position of cannula tips. (B) Preceding cocaine injections with intra-VTA infusions of AP5 blocks the induction of locomotor sensitization by cocaine in both WT (n = 8) and NR1DATCre (n = 5) mice, an effect that was not observed by intra-VTA saline injections in WT (n = 5) and NR1DATCre (n = 6). Data were collected on days 1 and 4 of cocaine treatment. The results show a significant difference between days 1 and 4 for the saline-infused animals (* p<0.001, two way ANOVA, day, F1,21 = 18.533), but not for those treated with intra-VTA AP5 (p = 0.679, two way ANOVA).
Figure 9. Development of cocaine-induced CPP, extinction, and reinstatement.
NR1DATCre (n = 8) and WT (n = 8) animals exhibit no significant difference between saline and cocaine-associated compartments before conditioning (A, pre-test) and significant conditioned place preference for the cocaine-associated compartment following conditioning (B). The data are expressed as the time in seconds spent in the saline and cocaine paired compartments during the pre-test (A), preference test (B), after extinction (C), and reinstatement (D) for WT and KO animals ± SEM. Cocaine dose was 10 mg/kg. Significant differences were found in time spent in cocaine-paired compartment as compared to pre-test (*), and time spent in cocaine-paired compartment as compared to saline-paired compartment within each test session (#). Two-factor repeated measures ANOVA, p≤0.05.
Similar articles
- Cocaine enhances NMDA receptor-mediated currents in ventral tegmental area cells via dopamine D5 receptor-dependent redistribution of NMDA receptors.
Schilström B, Yaka R, Argilli E, Suvarna N, Schumann J, Chen BT, Carman M, Singh V, Mailliard WS, Ron D, Bonci A. Schilström B, et al. J Neurosci. 2006 Aug 16;26(33):8549-58. doi: 10.1523/JNEUROSCI.5179-05.2006. J Neurosci. 2006. PMID: 16914681 Free PMC article. - Targeting VGLUT2 in Mature Dopamine Neurons Decreases Mesoaccumbal Glutamatergic Transmission and Identifies a Role for Glutamate Co-release in Synaptic Plasticity by Increasing Baseline AMPA/NMDA Ratio.
Papathanou M, Creed M, Dorst MC, Bimpisidis Z, Dumas S, Pettersson H, Bellone C, Silberberg G, Lüscher C, Wallén-Mackenzie Å. Papathanou M, et al. Front Neural Circuits. 2018 Aug 29;12:64. doi: 10.3389/fncir.2018.00064. eCollection 2018. Front Neural Circuits. 2018. PMID: 30210305 Free PMC article. - Cocaine Exposure Enhances the Activity of Ventral Tegmental Area Dopamine Neurons via Calcium-Impermeable NMDARs.
Creed M, Kaufling J, Fois GR, Jalabert M, Yuan T, Lüscher C, Georges F, Bellone C. Creed M, et al. J Neurosci. 2016 Oct 19;36(42):10759-10768. doi: 10.1523/JNEUROSCI.1703-16.2016. J Neurosci. 2016. PMID: 27798131 Free PMC article. - Cocaine-induced changes in NMDA receptor signaling.
Ortinski PI. Ortinski PI. Mol Neurobiol. 2014 Oct;50(2):494-506. doi: 10.1007/s12035-014-8636-6. Epub 2014 Jan 21. Mol Neurobiol. 2014. PMID: 24445951 Free PMC article. Review. - Synaptic and intrinsic plasticity in the ventral tegmental area after chronic cocaine.
Francis TC, Gantz SC, Moussawi K, Bonci A. Francis TC, et al. Curr Opin Neurobiol. 2019 Feb;54:66-72. doi: 10.1016/j.conb.2018.08.013. Epub 2018 Sep 17. Curr Opin Neurobiol. 2019. PMID: 30237117 Free PMC article. Review.
Cited by
- Calcium-permeable AMPA receptors in the VTA and nucleus accumbens after cocaine exposure: when, how, and why?
Wolf ME, Tseng KY. Wolf ME, et al. Front Mol Neurosci. 2012 Jun 27;5:72. doi: 10.3389/fnmol.2012.00072. eCollection 2012. Front Mol Neurosci. 2012. PMID: 22754497 Free PMC article. - Pontomesencephalic Tegmental Afferents to VTA Non-dopamine Neurons Are Necessary for Appetitive Pavlovian Learning.
Yau HJ, Wang DV, Tsou JH, Chuang YF, Chen BT, Deisseroth K, Ikemoto S, Bonci A. Yau HJ, et al. Cell Rep. 2016 Sep 6;16(10):2699-2710. doi: 10.1016/j.celrep.2016.08.007. Epub 2016 Aug 25. Cell Rep. 2016. PMID: 27568569 Free PMC article. - Distinctive Neuroanatomic Regions Involved in Cocaine-Induced Behavioral Sensitization in Mice.
Santos-Baldaia RD, Wuo-Silva R, Sanabria V, Baldaia MA, Yokoyama TS, Coppi AA, Hollais AW, Marinho EAV, Oliveira-Lima AJ, Longo BM. Santos-Baldaia RD, et al. Biomedicines. 2023 Jan 27;11(2):383. doi: 10.3390/biomedicines11020383. Biomedicines. 2023. PMID: 36830920 Free PMC article. - Dynamic regulation of midbrain dopamine neuron activity: intrinsic, synaptic, and plasticity mechanisms.
Morikawa H, Paladini CA. Morikawa H, et al. Neuroscience. 2011 Dec 15;198:95-111. doi: 10.1016/j.neuroscience.2011.08.023. Epub 2011 Aug 16. Neuroscience. 2011. PMID: 21872647 Free PMC article. Review. - Ventral Midbrain NTS1 Receptors Mediate Conditioned Reward Induced by the Neurotensin Analog, D-Tyr[11]neurotensin.
Rouibi K, Bose P, Rompré PP, Warren RA. Rouibi K, et al. Front Neurosci. 2015 Dec 22;9:470. doi: 10.3389/fnins.2015.00470. eCollection 2015. Front Neurosci. 2015. PMID: 26733785 Free PMC article.
References
- Dunn JM, Inderwies BR, Licata SC, Pierce RC. Repeated administration of AMPA or a metabotropic glutamate receptor agonist into the rat ventral tegmental area augments the subsequent behavioral hyperactivity induced by cocaine. Psychopharmacology (Berl) 2005;179:172–180. - PubMed
- Harris GC, Aston-Jones G. Critical role for ventral tegmental glutamate in preference for a cocaine-conditioned environment. Neuropsychopharmacology. 2003;28:73–76. - PubMed
- Harris GC, Wimmer M, Byrne R, Aston-Jones G. Glutamate-associated plasticity in the ventral tegmental area is necessary for conditioning environmental stimuli with morphine. Neuroscience. 2004;129:841–847. - PubMed
- Kalivas PW, Alesdatter JE. Involvement of N-methyl-D-aspartate receptor stimulation in the ventral tegmental area and amygdala in behavioral sensitization to cocaine. J Pharmacol Exp Ther. 1993;267:486–495. - PubMed
- Vezina P, Queen AL. Induction of locomotor sensitization by amphetamine requires the activation of NMDA receptors in the rat ventral tegmental area. Psychopharmacology (Berl) 2000;151:184–191. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources