Spatial memory consolidation is associated with induction of several lysine-acetyltransferase (histone acetyltransferase) expression levels and H2B/H4 acetylation-dependent transcriptional events in the rat hippocampus - PubMed (original) (raw)
Spatial memory consolidation is associated with induction of several lysine-acetyltransferase (histone acetyltransferase) expression levels and H2B/H4 acetylation-dependent transcriptional events in the rat hippocampus
Olivier Bousiges et al. Neuropsychopharmacology. 2010 Dec.
Abstract
Numerous genetic studies have shown that the CREB-binding protein (CBP) is an essential component of long-term memory formation, through its histone acetyltransferase (HAT) function. E1A-binding protein p300 and p300/CBP-associated factor (PCAF) have also recently been involved in memory formation. By contrast, only a few studies have reported on acetylation modifications during memory formation, and it remains unclear as to how the system is regulated during this dynamic phase. We investigated acetylation-dependent events and the expression profiles of these HATs during a hippocampus-dependent task taxing spatial reference memory in the Morris water maze. We found a specific increase in H2B and H4 acetylation in the rat dorsal hippocampus, while spatial memory was being consolidated. This increase correlated with the degree of specific acetylated histones enrichment on some memory/plasticity-related gene promoters. Overall, a global increase in HAT activity was measured during this memory consolidation phase, together with a global increase of CBP, p300, and PCAF expression. Interestingly, these regulations were altered in a model of hippocampal denervation disrupting spatial memory consolidation, making it impossible for the hippocampus to recruit the CBP pathway (CBP regulation and acetylated-H2B-dependent transcription). CBP has long been thought to be present in limited concentrations in the cells. These results show, for the first time, that CBP, p300, and PCAF are dynamically modulated during the establishment of a spatial memory and are likely to contribute to the induction of a specific epigenetic tagging of the genome for hippocampus-dependent (spatial) memory consolidation. These findings suggest the use of HAT-activating molecules in new therapeutic strategies of pathological aging, Alzheimer's disease, and other neurodegenerative disorders.
Figures
Figure 1
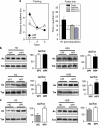
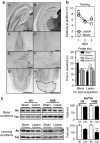
Specific increase of H2B/H4 acetylation levels in rat hippocampus during spatial memory formation. (a) Water maze performance in rats trained over 3 consecutive days (four trials per day) for spatial reference memory. Control rats had to swim toward a visible platform (VPf) whose location was changed from trial to trial. Learning rats had to swim to a hidden platform (HPf) at a fixed location. Left: Acquisition data are expressed as the mean (±SEM) distances to reach the platform. HPf rats showed a marked improvement between days 1 and 2; *p<0.001. Right: Probe trial performance in the HPf rats given as the mean time in the far quadrants (±SEM); performance was significantly above chance level (ie, 15 s) only in the target quadrant, whereas time in the three other quadrants was either at chance level or significantly under chance. *p<0.05, significantly different from chance. (b) Changes in histone acetylation levels during the formation of a spatial memory. Acetylated (Ac) and total (Tot) histone levels were measured by western blot analyses for each histone core in histone extracts obtained from the dorsal hippocampus of 3-day-trained rats (HPf vs VPf; _n_=7 per group). Lysine acetylations measured here are H3K9K14, H4K12, H2AK9, and H2BK5K12K15K20. Typical western blots are represented in duplicates. Quantified results are represented as fold induction of the Ac/Tot ratio for each histone, the ratio obtained in the control condition being arbitrarily set at 100%. Student's _t_-test: **p<0.01,*p<0.05, as compared with VPf group. Significant increases in acetylation of H2B (1.35-fold) and H4 (1.38-fold) levels, but not of H3 and H2A, were measured in the rat hippocampus after 3 days of spatial learning. (c) Histone acetylation level comparison between home cage (HC) vs VPf control rats. Rats were either trained in the visible version of the water maze (VPf, _n_=6) or left in their HC (_n_=5)—but manipulated daily for a few minutes—over 3 consecutive days. Histone extracts were prepared and the acetylated and total histone levels are shown in duplicate for H3K9K14 and H2BK5K12K15K20. A significant increase in H3 acetylation is measured between both groups (twofold), whereas H2B acetylation ratio remains unchanged. Student's _t_-test: **p<0.005, as compared with the HC group.
Figure 2
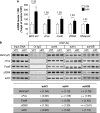
Several memory/plasticity-related genes present an acetylated-H2B/H4 enrichment on their promoters during the consolidation of a spatial memory. (a) mRNA expression levels of several CBP/memory-related genes: bdnf-eIV, cFos, FosB, and zif268 were evaluated by RT-qPCR in the dorsal hippocampus of control (visible platform, VPf) and learning (hidden platform, HPf) groups trained for 3 days. RNA polymerase II was tested as an internal negative control. Values were normalized to the 18S ribosomal subunit. Data are expressed as mean (±SEM) (_n_=6 per group), with percentage relative to the control group (VPf) arbitrarily set at 100%. Fold inductions are indicated above histograms. Student's _t_-test: *p<0.05, as compared with VPf. (b) Chromatin immunoprecipitation were performed on 3-day-trained rats from the VPf and HPf groups (_n_=3). Semiquantitative PCR were performed to analyze the presence of _bdnf-pIV_, _cFos_, _FosB_, and _zif268_ proximal promoters in the immunoprecipitates. Controls for immunoprecipitation were performed with an unrelated control antibody (Ct-IgG) or no antibody (noAb). Input DNA corresponding to the total of chromatin before immunoprecipitation was run in parallel as positive control. Representative results are shown in duplicate for each gene tested. _β_-Actin was used as internal control. Bands were quantified (ImageJ) and fold inductions (f.i.) are indicated in the table below. An activation score was arbitrarily affected by the HPf _vs_ VPf condition: (+++) when f.i. _>_2.00; (++) when f.i. _>_1.50; (+) when f.i. _>_1.25; and (=) when −0.25<f_.i.>+_0.25. acH3, acetylated K9, K14 H3 histone; acH4, acetylated K12 H4 histone; acH2B, acetylated K12, K15 H2B histone; Bdnf-eIV, bdnf exon IV; bdnf-pIV, bdnf promoter IV.
Figure 3
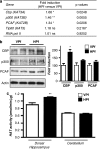
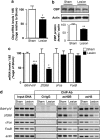
Increased expression level of several HATs and their activity during consolidation of a spatial memory. (a) HAT gene expression. mRNA expression levels of different genes were evaluated by RT-qPCR in the dorsal hippocampus of control (visible platform, VPf) and learning (hidden platform, HPf) groups (_n_=5 per group). Values were normalized to the 18S ribosomal subunit and the fold inductions and _p_-values are presented. Student's _t_-test: *p<0.05, **p<0.01, and NS, nonsignificant. Cbp, p300, and PCAF mRNA levels are significantly increased in the hippocampi of learners (HPf). CBP, CREB-binding protein; p300, E1A-binding protein p300; PCAF, p300/CBP-associated factor; Tip60, HIV-1 tat interactive protein; and RNA pol II, polymerase (RNA) II. The new nomenclature of HATs as lysine(K)-acetyltransferases (KATs) is mentioned within parenthesis (Allis et al, 2007). (b) HAT protein expression. HAT levels were assessed by western blot in nuclear protein extracts obtained from the dorsal hippocampus of rats trained as in (a). Typical blots are shown in duplicates. Blots were quantified (_n_=7 per group) and results normalized against actin are shown (right). *p<0.05, as compared with VPf. CBP was increased by 1.86-fold in the dorsal hippocampus during consolidation of spatial learning. P300 and PCAF show a nonsignificant trend to increase in the same conditions. (c) HAT activity. The same nuclear protein extracts as in (b) were used. HAT activity was measured on 30 μg of dorsal hippocampus or cerebellum nuclear protein extracts from the two rat groups (VPf and HPf). HAT activity is expressed in nmol/min/μg protein according to _A_=ɛLC. No significant change is found in the cerebellum, whereas global HAT activity was increased in the dorsal hippocampus. **p<0.01, HPf vs VPf.
Figure 4
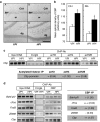
CBP-dependent regulations in the rat dorsal hippocampus during the formation of a spatial memory. (a) Representative images of CBP immunoreactive staining revealed with DAB staining in regions CA1 (A, B) and in the dentate gyrus (dg; C, D) of the dorsal hippocampus in representative VPf rats (A, C) and HPf (B, D) rats. Pyr: pyramidal neuron layer; sr: stratum radiatum; dg: dentate gyrus. Scale bar=250 μm. (b) Mean (±SEM) optical density of CBP immunoreactive staining in the CA1 pyramidal cell layer (CA1) and in the dentate gyrus granular layer (DG). In HPf rats (_n_=5), whatever the region, the optical density of CBP immunoreactivity was significantly larger than in VPf rats (_n_=5). *p<0.05. (c) Chromatin immunoprecipitation of acetylated histones were performed on chromatin obtained from 3-day-trained rats from the VPf and HPf groups (_n_=3). Semiquantitative PCR were performed to analyze the presence of _cbp_ proximal promoter in the immunoprecipitates. Controls for immunoprecipitation were performed with an unrelated control antibody (Ct-IgG) or no antibody (noAb, not shown). Representative results are shown in duplicate for each gene tested. Bands were quantified (ImageJ) and fold inductions (f.i.) are indicated in the table below. acH3: acetylated K9, K14 H3 histone; acH4: acetylated K12 H4 histone; and acH2B: acetylated K12, K15 H2B histone. (d) Chromatin immunoprecipitation of CBP were performed on chromatin obtained from 3-day-trained rats from the VPf and HPf groups (_n_=3). Semiquantitative PCR were performed to analyze the presence of _bdnf-pIV_, _cFos_, _FosB_, _zif268_, and _cbp_ proximal promoters in the immunoprecipitates. Controls for immunoprecipitation were performed with an unrelated control antibody (Ct-IgG, shown in simplicate) or no antibody (noAb, not shown). Representative results are shown in duplicate for each gene tested. Bands were quantified (ImageJ) and fold inductions (f.i.) are indicated in the table on the right. _Bdnf-eIV_: _bdnf_ exon IV; _bdnf-pIV_: _bdnf_ promoter IV. (c, d) Input DNA corresponding to the total of chromatin before immunoprecipitation was run in parallel as positive control. An activation score was arbitrarily affected by the HPf _vs_ VPf condition: (+++) when f.i. >2.00; (++) when f.i.>1.50; (+) when f.i. >1.25; (=) when 1.25<f.i.>0.75; and (−) when f.i.<0.75.
Figure 5
Histone acetylation levels in a cognitively impaired rat model. (a) Histological verification of the location and extent of cholinergic and entorhinal cortex (EC) lesions. A, C, E, and G are representative pictures of one sham-operated rat; B, D, F, and H are representative pictures of one rat subjected to the double lesions. A, B are typical examples of AChE-positive staining in the posterior hippocampus as observed at about −5.5 mm from Bregma (notice the marked decrease of dark staining accounting for cholinergic denervation in B); C, D are typical examples of ChAT-positive immunostaining in the medial septum at about +0.2 mm from Bregma (notice the dramatic reduction in D); E, F are examples of ChAT-positive immunostaining in the nucleus basalis magnocellularis and the substantia innominata (notice that the decrease is much less pronounced than in the septal region in F). G, H are examples of NeuN-positive staining in the posterior region of the cortex (coronal sections) illustrating the lesion extent in the medial and lateral entorhinal cortex at about Bregma 7.6 mm (notice the almost total loss of neuronal staining within the area delimited by the stippled line in H). All coordinates indicated in this caption are according to Paxinos and Watson (1998). Scale bar=1000 μm in A, B, G, and H, and scale bar=500 μm in C, D, E, and F. (b) Effects of double lesions on water maze performance. Upper panel: Acquisition data during the 3-day-training period are expressed as the mean (±SEM) of the distances to reach the platform in rats subjected to a lesion of septal cholinergic neurons and a fiber-sparing lesion of neurons in the EC (lesion, _n_=10) as compared with performance in the sham-operated controls (sham, _n_=11). Lower panel: Probe trial performance indicated as the mean time in the far quadrants (±SEM). Sham-operated control rats showed a performance in the target quadrant that was significantly above chance (ie, 15 s). In contrast, the double lesion affected spatial memory functions, as the lesioned rats did not focus their search in the appropriate quadrant. *p<0.05, significantly above chance. (c) Acetylated (Ac) and total (Tot) histone levels were measured by western blot analyses for H3 and H2B histones in total extracts obtained from the dorsal hippocampus of home cage rats (basal conditions; sham, _n_=6; lesion, _n_=8) or of trained rats (learning conditions: HPf group during 3 days for all rat groups; sham, _n_=7; lesion, _n_=7). Lysine acetylations measured are H3K9K14 and H2BK5K12K15K20. Typical western blots are represented in duplicates. Quantified results are represented as fold induction of the Ac/Tot ratio for each histone. The ratio obtained in the control condition is arbitrarily set at 100%. Sh, sham; Les, lesion. Student's _t_-test: **p<0.01. A significant decrease in acetylation of H2B is observed in the hippocampus of trained rats, but not in that of home cage rats. Acetylated-H3 levels tend to decrease as well only in learning conditions.
Figure 6
CBP- and histone acetylation-dependent regulations during formation of a spatial memory in a cognitively impaired rat model. Cbp transcripts (a) were evaluated by real-time RT-PCR (_n_=5) and CBP protein (b) by western blot analyses (_n_=7) in the dorsal hippocampus from sham-operated (sham) or double lesion (lesion) rats group after a 3-day-training period. CBP levels were not increased by the spatial memory training in lesion rats, *p<0.05, **p<0.01, as compared with sham, (c) Real-time RT-PCR analysis of bdnf-eIV, _zif26_8, cFos, and FosB genes in the same conditions as in (a), *p<0.05, ***p<0.001, as compared with sham (_n_=5). (d) Chromatin immunoprecipitation were performed on 3-day-trained rats from sham and lesion groups in the HPf version of the water maze (_n_=3 per group). Semiquantitative PCR were performed to analyze the presence of bdnf-pIV, cFos, FosB, and zif268 proximal promoters in the immunoprecipitates. Controls for immunoprecipitation were performed with an unrelated control antibody (Ct-IgG) or no antibody (noAb). Input DNA corresponding to the total of chromatin before immunoprecipitation was run in parallel as positive control. Representative results are shown in duplicate for each gene tested. _β_-Actin was used as internal control. acH3: acetylated K9, K14 H3 histone; acH2B: acetylated K12, K15 H2B histone; bdnf-eIV: bdnf exon IV; and bdnf-pIV: bdnf promoter IV. After 3 days of spatial training, none of the promoters are enriched in acetylated-H2B or in acetylated-H3 histones in the lesion rat group as compared with sham-operated rats.
Figure 7
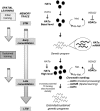
Hypothetical model of molecular interplay underlying consolidation of spatial memory. During the initial steps of a training aimed at acquiring a spatial task, the memory trace is progressively setup following a cascade of intracellular signals involved in short-term memory (STM) processing. These processes are non-transcriptional events (Kandel, 2001; Medina et al, 2008) as existing co-activators (HATs, basal levels) and transcription factors present in neurons are likely in sufficient quantities to promote a genetic program necessary for early consolidation signals. Here we show that, while training is pursued, the expression of several HATs (CBP, p300, and p300/CBP-associated factor (PCAF)) is increased, thus generating new HAT molecules (HATs, high level)—and CBP in particular—during a critical time window of spatial memory formation. We propose that reaching a new HAT threshold could allow a widening of HAT-binding partners to a larger panel of transcription factors and/or increase HAT stability over time, thereby potently triggering extended and/or sustained specific genetic programs for late consolidation. We showed that repetition of training (‘sustained training') is associated with increased HAT activity and new chromatin marking: histone H3 is acetylated in response to context-related processing (visible platform), whereas H2B histone is acetylated during spatial learning (hidden platform). H4 histone acetylation is also increased during the late consolidation phase. Each acetylation step can be reversed under the action of histone deacetylase (HDAC) enzymes, probably underlying the memory enhancer effect of some HDAC inhibitors (HDACi) (for reviews see, Barrett and Wood, 2008). Taken together, our results suggest that an epigenetic tagging of the genome takes place under the influence of HAT expression at specific memory/plasticity genes throughout the formation of spatial long-term memory (LTM). They also emphasize that directly promoting this chromatin marking pattern with HAT activators could be a new therapeutic strategy for memory enhancers, potently more specific than HDAC inhibition. Ac, acetylated; hist., histone; HATa, HAT activator; TF, transcription factor.
Similar articles
- Dissociable roles for histone acetyltransferases p300 and PCAF in hippocampus and perirhinal cortex-mediated object memory.
Mitchnick KA, Creighton SD, Cloke JM, Wolter M, Zaika O, Christen B, Van Tiggelen M, Kalisch BE, Winters BD. Mitchnick KA, et al. Genes Brain Behav. 2016 Jul;15(6):542-57. doi: 10.1111/gbb.12303. Genes Brain Behav. 2016. PMID: 27251651 - Vitamin A deficiency impairs spatial learning and memory: the mechanism of abnormal CBP-dependent histone acetylation regulated by retinoic acid receptor alpha.
Hou N, Ren L, Gong M, Bi Y, Gu Y, Dong Z, Liu Y, Chen J, Li T. Hou N, et al. Mol Neurobiol. 2015 Apr;51(2):633-47. doi: 10.1007/s12035-014-8741-6. Epub 2014 May 24. Mol Neurobiol. 2015. PMID: 24859384 - Hippocampal focal knockout of CBP affects specific histone modifications, long-term potentiation, and long-term memory.
Barrett RM, Malvaez M, Kramar E, Matheos DP, Arrizon A, Cabrera SM, Lynch G, Greene RW, Wood MA. Barrett RM, et al. Neuropsychopharmacology. 2011 Jul;36(8):1545-56. doi: 10.1038/npp.2011.61. Epub 2011 Apr 20. Neuropsychopharmacology. 2011. PMID: 21508930 Free PMC article. - Histone acetyl transferases as emerging drug targets.
Dekker FJ, Haisma HJ. Dekker FJ, et al. Drug Discov Today. 2009 Oct;14(19-20):942-8. doi: 10.1016/j.drudis.2009.06.008. Epub 2009 Jul 2. Drug Discov Today. 2009. PMID: 19577000 Review. - Transcriptional/epigenetic regulator CBP/p300 in tumorigenesis: structural and functional versatility in target recognition.
Wang F, Marshall CB, Ikura M. Wang F, et al. Cell Mol Life Sci. 2013 Nov;70(21):3989-4008. doi: 10.1007/s00018-012-1254-4. Epub 2013 Jan 11. Cell Mol Life Sci. 2013. PMID: 23307074 Free PMC article. Review.
Cited by
- Proteasome regulates transcription-favoring histone methylation, acetylation and ubiquitination in long-term synaptic plasticity.
Bach SV, Tacon PR, Morgan JW, Hegde AN. Bach SV, et al. Neurosci Lett. 2015 Mar 30;591:59-64. doi: 10.1016/j.neulet.2015.02.029. Epub 2015 Feb 14. Neurosci Lett. 2015. PMID: 25687290 Free PMC article. - HDAC inhibition facilitates the switch between memory systems in young but not aged mice.
Dagnas M, Guillou JL, Prévôt T, Mons N. Dagnas M, et al. J Neurosci. 2013 Jan 30;33(5):1954-63. doi: 10.1523/JNEUROSCI.3453-12.2013. J Neurosci. 2013. PMID: 23365234 Free PMC article. - Environmental Enrichment Induces Changes in Long-Term Memory for Social Transmission of Food Preference in Aged Mice through a Mechanism Associated with Epigenetic Processes.
Cintoli S, Cenni MC, Pinto B, Morea S, Sale A, Maffei L, Berardi N. Cintoli S, et al. Neural Plast. 2018 Jul 16;2018:3725087. doi: 10.1155/2018/3725087. eCollection 2018. Neural Plast. 2018. PMID: 30123245 Free PMC article. - Acetyltransferases (HATs) as targets for neurological therapeutics.
Schneider A, Chatterjee S, Bousiges O, Selvi BR, Swaminathan A, Cassel R, Blanc F, Kundu TK, Boutillier AL. Schneider A, et al. Neurotherapeutics. 2013 Oct;10(4):568-88. doi: 10.1007/s13311-013-0204-7. Neurotherapeutics. 2013. PMID: 24006237 Free PMC article. Review. - Epigenetic control of learning and memory in Drosophila by Tip60 HAT action.
Xu S, Wilf R, Menon T, Panikker P, Sarthi J, Elefant F. Xu S, et al. Genetics. 2014 Dec;198(4):1571-86. doi: 10.1534/genetics.114.171660. Epub 2014 Oct 17. Genetics. 2014. PMID: 25326235 Free PMC article.
References
- Alarcon JM, Malleret G, Touzani K, Vronskaya S, Ishii S, Kandel ER, et al. Chromatin acetylation, memory, and LTP are impaired in CBP+/− mice: a model for the cognitive deficit in Rubinstein–Taybi syndrome and its amelioration. Neuron. 2004;42:947–959. - PubMed
- Allis CD, Berger SL, Cote J, Dent S, Jenuwien T, Kouzarides T, et al. New nomenclature for chromatin-modifying enzymes. Cell. 2007;131:633–636. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous