H2AX Phosphorylation: Its Role in DNA Damage Response and Cancer Therapy - PubMed (original) (raw)
H2AX Phosphorylation: Its Role in DNA Damage Response and Cancer Therapy
Monika Podhorecka et al. J Nucleic Acids. 2010.
Abstract
Double-strand breaks (DSBs) are the most deleterious DNA lesions, which, if left unrepaired, may have severe consequences for cell survival, as they lead to chromosome aberrations, genomic instability, or cell death. Various physical, chemical, and biological factors are involved in DSB induction. Cells respond to DNA damage by activating the so-called DNA damage response (DDR), a complex molecular mechanism developed to detect and repair DNA damage. The formation of DSBs triggers activation of many factors, including phosphorylation of the histone variant H2AX, producing gammaH2AX. Phosphorylation of H2AX plays a key role in DDR and is required for the assembly of DNA repair proteins at the sites containing damaged chromatin as well as for activation of checkpoints proteins which arrest the cell cycle progression. In general, analysis of gammaH2AX expression can be used to detect the genotoxic effect of different toxic substances. When applied to clinical samples from cancer patients, evaluation of gammaH2AX levels may allow not only to monitor the efficiency of anticancer treatment but also to predict of tumor cell sensitivity to DNA damaging anticancer agents and toxicity of anticancer treatment toward normal cells.
Figures
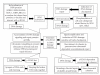
Figure 1
H2AX phosphorylation and its role in DNA damage response upon phosphorylation H2AX became critical player in DNA damage response. Activity of _γ_H2AX could be summarized to following main points: accumulation of DNA damage signaling and repair proteins at DSBs The generation of DSBs triggers the relocalization of many DNA damage response (DDR) proteins such as MRE11/NBS1/RAD50, MDC1, 53BP1and BRCA1 to nuclear foci where these proteins colocalize and interact with _γ_H2AX. Signal amplification and induction of DNA damage-sensitive cell cycle checkpoints _γ_H2AX contribute to the recruitment of ATM to DSB sites and activation of ATM-dependent cell cycle checkpoints. Chromatin remodeling to prevent dissociation of break ends and enhance DSB processing and repair efficiency The _γ_H2AX foci help keeping the broken DNA ends together and make successful and faithful repair more likely. Recruitment of cohesins to the site of DNA damage to promote sister chromatid-dependent recombinational repair DNA cohesion induced by double-strand DNA break and mediated by _γ_H2AX has an important function during repair of double-strand breaks following DNA replication by holding the damaged chromatid close to its undamaged sister template.
Similar articles
- [gamma H2AX in the recognition of DNA double-strand breaks].
Podhorecka M. Podhorecka M. Postepy Hig Med Dosw (Online). 2009 Feb 27;63:92-8. Postepy Hig Med Dosw (Online). 2009. PMID: 19252467 Review. Polish. - H2AX phosphorylation at the sites of DNA double-strand breaks in cultivated mammalian cells and tissues.
Firsanov DV, Solovjeva LV, Svetlova MP. Firsanov DV, et al. Clin Epigenetics. 2011 Aug;2(2):283-97. doi: 10.1007/s13148-011-0044-4. Epub 2011 Jun 25. Clin Epigenetics. 2011. PMID: 22704343 Free PMC article. - The loss of gammaH2AX signal is a marker of DNA double strand breaks repair only at low levels of DNA damage.
Bouquet F, Muller C, Salles B. Bouquet F, et al. Cell Cycle. 2006 May;5(10):1116-22. doi: 10.4161/cc.5.10.2799. Epub 2006 May 15. Cell Cycle. 2006. PMID: 16721046 - 111In-Labeled DTPA-conjugated Tat-linked anti-phosphorylated histone protein H2AX antibody.
Chopra A. Chopra A. 2011 Jul 28 [updated 2011 Aug 25]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. 2011 Jul 28 [updated 2011 Aug 25]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. PMID: 21882403 Free Books & Documents. Review. - H2AX: A key player in DNA damage response and a promising target for cancer therapy.
Prabhu KS, Kuttikrishnan S, Ahmad N, Habeeba U, Mariyam Z, Suleman M, Bhat AA, Uddin S. Prabhu KS, et al. Biomed Pharmacother. 2024 Jun;175:116663. doi: 10.1016/j.biopha.2024.116663. Epub 2024 Apr 30. Biomed Pharmacother. 2024. PMID: 38688170 Review.
Cited by
- Hydro-alcoholic Extract of Achillea Wilhelmsii C. Koch Reduces the Expression of Cell Death-Associated Genes while Inducing DNA Damage in HeLa Cervical Cancer Cells.
Sargazi S, Moudi M, Kooshkaki O, Mirinejad S, Saravani R. Sargazi S, et al. Iran J Med Sci. 2020 Sep;45(5):359-367. doi: 10.30476/ijms.2020.72657.0. Iran J Med Sci. 2020. PMID: 33060879 Free PMC article. - Antitumor Activity of a 5-Hydroxy-1H-Pyrrol-2-(5H)-One-Based Synthetic Small Molecule In Vitro and In Vivo.
Geng Y, Wang X, Yang L, Sun H, Wang Y, Zhao Y, She R, Wang MX, Wang DX, Tang J. Geng Y, et al. PLoS One. 2015 Jun 4;10(6):e0128928. doi: 10.1371/journal.pone.0128928. eCollection 2015. PLoS One. 2015. PMID: 26042776 Free PMC article. - Polyphenolic Platform Ameliorated Sanshool for Skin Photoprotection.
Wang T, Guo L, Wu S, Xu Y, Song J, Yang Y, Zhang H, Li D, Li Y, Jiang X, Gu Z. Wang T, et al. Adv Sci (Weinh). 2024 Apr;11(16):e2310012. doi: 10.1002/advs.202310012. Epub 2024 Feb 15. Adv Sci (Weinh). 2024. PMID: 38359060 Free PMC article. - Loss of Dystroglycan Drives Cellular Senescence via Defective Mitosis-Mediated Genomic Instability.
Jimenez-Gutierrez GE, Mondragon-Gonzalez R, Soto-Ponce LA, Gómez-Monsiváis WL, García-Aguirre I, Pacheco-Rivera RA, Suárez-Sánchez R, Brancaccio A, Magaña JJ, C R Perlingeiro R, Cisneros B. Jimenez-Gutierrez GE, et al. Int J Mol Sci. 2020 Jul 14;21(14):4961. doi: 10.3390/ijms21144961. Int J Mol Sci. 2020. PMID: 32674290 Free PMC article.
References
- Jackson SP. Sensing and repairing DNA double-strand breaks. Carcinogenesis. 2002;23(5):687–696. - PubMed
- Sonoda E, Hochegger H, Saberi A, Taniguchi Y, Takeda S. Differential usage of non-homologous end-joining and homologous recombination in double strand break repair. DNA Repair. 2006;5(9-10):1021–1029. - PubMed
- Smider V, Chu G. The end-joining reaction in V(D)J recombination. Seminars in Immunology. 1997;9(3):189–197. - PubMed
- Jackson SP. Detecting, signalling and repairing DNA double-strand breaks. Biochemical Society Transactions. 2001;29(6):655–661. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources