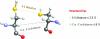Redox biology: computational approaches to the investigation of functional cysteine residues - PubMed (original) (raw)
Review
Redox biology: computational approaches to the investigation of functional cysteine residues
Stefano M Marino et al. Antioxid Redox Signal. 2011.
Abstract
Cysteine (Cys) residues serve many functions, such as catalysis, stabilization of protein structure through disulfides, metal binding, and regulation of protein function. Cys residues are also subject to numerous post-translational modifications. In recent years, various computational tools aiming at classifying and predicting different functional categories of Cys have been developed, particularly for structural and catalytic Cys. On the other hand, given complexity of the subject, bioinformatics approaches have been less successful for the investigation of regulatory Cys sites. In this review, we introduce different functional categories of Cys residues. For each category, an overview of state-of-the-art bioinformatics methods and tools is provided, along with examples of successful applications and potential limitations associated with each approach. Finally, we discuss Cys-based redox switches, which modify the view of distinct functional categories of Cys in proteins.
Figures
FIG. 1.
Different functional categories of Cys in proteins. Schematic representation of different functions of Cys residues discussed in this review. In addition, Cys redox switches may belong to more than one functional category. To illustrate this concept, a circle with arrows is shown that connects various Cys functions. (To see this illustration in color the reader is referred to the web version of this article at
).
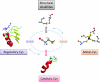
FIG. 2.
Structure-based method for predicting metal-binding Cys. This figure illustrates a case of Cys and Zn coordination (Cys4–Zn). (A) First part of the Fold-X approach for Cys-Zn site prediction. By analyzing all coordination complexes between Cys(Sγ) and Zn atoms in the PDB repository, a table of geometrical values for Zn–Cys sites is compiled. Different Zn–Cys binding modes, found in the PDB, are shown (Zn atoms are represented as a cloud of cyan balls). The center of gravity of the cloud is calculated (dark blue ball), representing the canonical binding mode (CBM) in Cys4–Zn sites. (B) Whenever a query protein structure is analyzed by the algorithm, each potential Cys is screened for CBMs: if clusters of 4 CBMs are found (e.g., 4 spatially close Cys, with at least partially super-imposable CBMs, shown as blue balls), a Cys4–Zn metal-binding site is predicted. As a final step, the binding site is subjected to geometrical optimization using Fold-X. After minimization, final coordinates for the predicted Cys4–Zn binding site are obtained (red ball). (To see this illustration in color the reader is referred to the web version of this article at
).
FIG. 3.
Structure-based prediction of disulfide bonds. Two commonly used approaches for the determination of disulfides in protein structures are shown. The first employs sulfur to sulfur distance (S–S distance in the figure) of ≤2.5 Å. In the second approach, the distance between α-carbons of two proximal Cys (C α –C α distance in the figure) is measured. When the C α –C α distance is ≤8 Å, the two Cys residues are considered disulfide bonded. (To see this illustration in color the reader is referred to the web version of this article at
).
FIG. 4.
Methods for prediction of thiol oxidoreductases. (A) Sec/Cys method. A query protein is analyzed (with tBlastn) against a database of nucleotide sequences containing all potential selenoproteins (e.g., all nucleotide sequences in NCBI). Sec pairing with Cys, flanked by conserved sequences, leads to the prediction of Cys function (i.e., the Cys aligning with Sec is predicted to serve redox function). U is Sec. (B) Structure-based prediction of thiol oxidoreductases. By analyzing (i) sequence and structural homology with known thiol oxidoreductases (knowledge-based information, descriptions preceded by red bullets in the right side of panel B), and (ii) chemical and physical activation of its functional groups (energy-based information, descriptions preceded by blue bullets in the right side of panel B), a query protein is evaluated. Combining the two independent types of information, true positives can be detected even with very little sequence similarity to known thiol oxidoreductases, and additional candidate thiol oxidoreductases can be predicted. (To see this illustration in color the reader is referred to the web version of this article at
).
FIG. 5.
Effects of S-nitrosylation on Cys force field parameters for predicting trans-nitrosylation sites. Ad hoc charge scheme for NO-Cys is shown. R1 and R2 stand for generic substituents. These parameters were applied and validated both for NO-Cys in proteins (where R1 and R2 are adjacent amino acids) and generic organic molecules (where R1 and R2 are –CH2CH3 moieties). After nitrosylation, significant charge relocation occurs in side chain atoms, particularly affecting the sulfur (most of its negative charge relocates to the terminal oxygen atom, OE in the scheme). The ad hoc parameters can be transferred to any Cys-containing molecule; for example, starting from glutathione (GSH), in silico S-nitrosylation can be simulated with a molecular builder tool, constructing the S-nitrosoglutathione (GSNO) molecule. To deal with the modification, ad hoc parameters for NO-Cys are transferred to GSNO (by keeping the overall charge of GSNO fixed, such that only partial charge redistribution can occur). For predictive purposes, GSNO can then be docked to a query protein with docking algorithms. For each Cys of the query protein, affinity for GSNO is calculated. Cys showing favorable energetic and geometrical interaction with GSNO are predicted as potential modification sites. By analogy, many other potential trans-nitrosylating agents can be tested (e.g., Cys-NO, NO-Cys containing peptides) with similar docking-based approaches. (To see this illustration in color the reader is referred to the web version of this article at
).
Similar articles
- Cy-preds: An algorithm and a web service for the analysis and prediction of cysteine reactivity.
Soylu İ, Marino SM. Soylu İ, et al. Proteins. 2016 Feb;84(2):278-91. doi: 10.1002/prot.24978. Proteins. 2016. PMID: 26685111 - ROSics: chemistry and proteomics of cysteine modifications in redox biology.
Kim HJ, Ha S, Lee HY, Lee KJ. Kim HJ, et al. Mass Spectrom Rev. 2015 Mar-Apr;34(2):184-208. doi: 10.1002/mas.21430. Epub 2014 Jun 10. Mass Spectrom Rev. 2015. PMID: 24916017 Free PMC article. Review. - Analysis of Cysteine Redox Post-Translational Modifications in Cell Biology and Drug Pharmacology.
Wani R, Murray BW. Wani R, et al. Methods Mol Biol. 2017;1558:191-212. doi: 10.1007/978-1-4939-6783-4_9. Methods Mol Biol. 2017. PMID: 28150239 - Characterization of surface-exposed reactive cysteine residues in Saccharomyces cerevisiae.
Marino SM, Li Y, Fomenko DE, Agisheva N, Cerny RL, Gladyshev VN. Marino SM, et al. Biochemistry. 2010 Sep 7;49(35):7709-21. doi: 10.1021/bi100677a. Biochemistry. 2010. PMID: 20698499 Free PMC article. - Structural, redox, and mechanistic parameters for cysteine-sulfenic acid function in catalysis and regulation.
Claiborne A, Mallett TC, Yeh JI, Luba J, Parsonage D. Claiborne A, et al. Adv Protein Chem. 2001;58:215-76. doi: 10.1016/s0065-3233(01)58006-7. Adv Protein Chem. 2001. PMID: 11665489 Review.
Cited by
- Sulfolobus solfataricus thiol redox puzzle: characterization of an atypical protein disulfide oxidoreductase.
Limauro D, De Simone G, Pirone L, Bartolucci S, D'Ambrosio K, Pedone E. Limauro D, et al. Extremophiles. 2014 Mar;18(2):219-28. doi: 10.1007/s00792-013-0607-8. Epub 2013 Dec 5. Extremophiles. 2014. PMID: 24306780 - Thiol-Based Redox Modulation of Soluble Guanylyl Cyclase, the Nitric Oxide Receptor.
Beuve A. Beuve A. Antioxid Redox Signal. 2017 Jan 20;26(3):137-149. doi: 10.1089/ars.2015.6591. Epub 2016 Apr 1. Antioxid Redox Signal. 2017. PMID: 26906466 Free PMC article. Review. - Redox Paradox: A Novel Approach to Therapeutics-Resistant Cancer.
Chaiswing L, St Clair WH, St Clair DK. Chaiswing L, et al. Antioxid Redox Signal. 2018 Nov 1;29(13):1237-1272. doi: 10.1089/ars.2017.7485. Epub 2018 Feb 21. Antioxid Redox Signal. 2018. PMID: 29325444 Free PMC article. Review. - pCysMod: Prediction of Multiple Cysteine Modifications Based on Deep Learning Framework.
Li S, Yu K, Wu G, Zhang Q, Wang P, Zheng J, Liu ZX, Wang J, Gao X, Cheng H. Li S, et al. Front Cell Dev Biol. 2021 Feb 23;9:617366. doi: 10.3389/fcell.2021.617366. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 33732693 Free PMC article. - Thioredoxin 1-mediated post-translational modifications: reduction, transnitrosylation, denitrosylation, and related proteomics methodologies.
Wu C, Parrott AM, Fu C, Liu T, Marino SM, Gladyshev VN, Jain MR, Baykal AT, Li Q, Oka S, Sadoshima J, Beuve A, Simmons WJ, Li H. Wu C, et al. Antioxid Redox Signal. 2011 Nov 1;15(9):2565-604. doi: 10.1089/ars.2010.3831. Epub 2011 Jun 8. Antioxid Redox Signal. 2011. PMID: 21453190 Free PMC article. Review.
References
- Abkevich VI. Shakhnovich EI. What can disulfide bonds tell us about protein energetics, function and folding: Simulations and bioinformatics analysis. J Mol Biol. 2000;300:975–985. - PubMed
- Andreini C. Bertini I. Rosato A. A hint to search for metalloproteins in gene banks. Bioinformatics. 2004;20:1373–1380. - PubMed
- Azevedo D. Tacnet F. Delaunay A. Rodrigues–Pousada C. Toledano MB. Two redox centers within Yap1 for H2O2 and thiol-reactive chemicals signaling. Free Radic Biol Med. 2003;35:889–900. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources