Translocation of Crohn's disease Escherichia coli across M-cells: contrasting effects of soluble plant fibres and emulsifiers - PubMed (original) (raw)
Translocation of Crohn's disease Escherichia coli across M-cells: contrasting effects of soluble plant fibres and emulsifiers
Carol L Roberts et al. Gut. 2010 Oct.
Abstract
Background: Crohn's disease is common in developed nations where the typical diet is low in fibre and high in processed food. Primary lesions overlie Peyer's patches and colonic lymphoid follicles where bacterial invasion through M-cells occurs. We have assessed the effect of soluble non-starch polysaccharide (NSP) and food emulsifiers on translocation of Escherichia coli across M-cells.
Methods: To assess effects of soluble plant fibres and food emulsifiers on translocation of mucosa-associated E coli isolates from Crohn's disease patients and from non-Crohn's controls, we used M-cell monolayers, generated by co-culture of Caco2-cl1 and Raji B cells, and human Peyer's patches mounted in Ussing chambers.
Results: E coli translocation increased across M-cells compared to parent Caco2-cl1 monocultures; 15.8-fold (IQR 6.2-32.0) for Crohn's disease E coli (N=8) and 6.7-fold (IQR 3.7-21.0) for control isolates (N=5). Electron microscopy confirmed E coli within M-cells. Plantain and broccoli NSP markedly reduced E coli translocation across M-cells at 5 mg/ml (range 45.3-82.6% inhibition, p<0.01); apple and leek NSP had no significant effect. Polysorbate-80, 0.01% vol/vol, increased E coli translocation through Caco2-cl1 monolayers 59-fold (p<0.05) and, at higher concentrations, increased translocation across M-cells. Similarly, E coli translocation across human Peyer's patches was reduced 45±7% by soluble plantain NSP (5 mg/ml) and increased 2-fold by polysorbate-80 (0.1% vol/vol).
Conclusions: Translocation of E coli across M-cells is reduced by soluble plant fibres, particularly plantain and broccoli, but increased by the emulsifier Polysorbate-80. These effects occur at relevant concentrations and may contribute to the impact of dietary factors on Crohn's disease pathogenesis.
Conflict of interest statement
Competing interests: JMR is a past/present member of advisory boards for Procter & Gamble, Schering-Plough, Chiesi, Falk and Celltech. With the University of Liverpool and Provexis Plc (UK), JMR holds a patent for use of a soluble fibre preparation as therapy for Crohn's disease. NO'K is an employee of Provexis Plc.
Figures
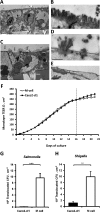
Figure 1
Co-culture model of M-cells. (A) Low power and (B) high power TEM images of representative control Caco2-cl1 monolayers, showing closely packed uniform microvilli on the apical aspect of the cell. (C) Low power and (D and E) high power images of in vitro derived M-cells. The M-cells possess an apical surface characteristically devoid of microvilli (indicated by black arrows). In images (A) and (C), the Millicell-PCF filter support can be seen on the lower portion of the image, as can the 3 μm pores within the filter (indicated by white arrows). Bar either 10 μm (A and C) or 1 μm (B, D and E). (F). TEER over time in Caco2-cl1 and M-cell monolayers. Monolayer TEER steadily increases during culture, indicating formation of fully confluent, differentiated monolayers (TEER values in excess of 300 Ω cm2). Monolayer incubation with Raji B cells in the lower chamber occurred on day 16 (dashed line) and continued for the following 5 days, leading to the formation of M-cells, while control Caco2-cl1 monolayers formed in the absence of Raji B cells. The TEER of the control Caco2-cl1 monolayers continued to increase over the following 5 days of incubation (day 16 to 21; solid boxes), as did the TEER of the M-cell monolayers (open boxes), although to a lesser extent (n=35 culture wells for each group). (G) Salmonella typhimurium and (H) Shigella sonnei show significantly greater translocation through M-cells than through Caco2-cl1 monolayers (N=3). **, p<0.01 and ***, p<0.001; Mann–Whitney U.
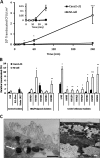
Figure 2
Translocation of human mucosal E coli isolates across M-cells. (A) Translocation across M-cell monolayers was first quantifiable within 15 min post-infection, while translocation across Caco2-cl1 monolayers could not be detected until at least 30 min post-infection. Data presented are for adherent, invasive Crohn's disease E coli HM605. (*, p<0.05 and ***, p<0.001; ANOVA; N=3). (B) Translocation is measured as CFU expressed relative to M-cell translocation of E coli K12 (N=8, with minimum n=8 replicates for each E coli). Crohn's disease E coli isolates were translocated through M-cell monolayers more readily than through Caco2-cl1 monolayers. Adherent, invasive control patient (irritable bowel syndrome/sporadic polyposis) E coli isolates also translocated through M-cell monolayers more readily, with the exception of isolate HM463. Neither E coli K12 nor E coli XL-1blue showed significantly better translocation through M-cells than through parent Caco2-cl1 cells. Translocation of the probiotic E coli Nissle 1917 was also very low through M-cells, and reduced compared to its translocation through Caco2-cl1 cells. *, p<0.05; **, p<0.01; and ***, p<0.001; ANOVA. (C) TEM showing the presence of intracellular E coli HM605 within M-cells (white arrows). Intracellular E coli HM605 were not seen within Caco2-cl1 monolayers. Bar=0.5 μm.
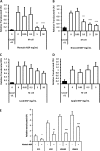
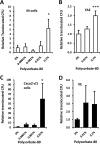
Figure 3
Plantain NSP blocks translocation of E coli across M-cells in vitro. (A) E coli HM605 translocation through M-cells is inhibited by the presence of plantain NSP at 5 mg/ml and 50 mg/ml (N=6). (B) Broccoli NSP inhibits bacterial translocation across M-cells at 0.5, 5 and 50 mg/ml (N=3). (C) Neither leek NSP nor (D) apple NSP inhibited HM605 translocation across M-cells (both N=2, each at least n=5 replicates). Translocation is measured as CFU expressed relative to M-cell translocation in the absence of fibres. (E) Plantain NSP (5 mg/ml) inhibits translocation across M-cells for a wide range of E coli (N=3). Translocation is measured as CFU expressed relative to M-cell translocation of E coli K12. For all, *, p<0.05; **, p<0.01; ***, p<0.001; ANOVA.
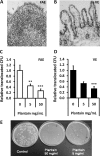
Figure 4
Plantain NSP blocks translocation of E coli across the human intestinal epithelium in Ussing chambers. Histology of (A) an ileal lymphoid follicle (LF) and overlying follicule associated epithelium (FAE) and of (B) villus epithelium (VE) following Ussing chamber experiments. ×20 magnification. (C and D) EGFP-expressing E coli HM615 translocation through both FAE (N=7) and VE (N=9) and is inhibited by the presence of plantain NSP. **p<0.01; ***, p<0.001; ANOVA. (E) Overnight culture of Ussing chamber serosal medium following 2 h translocation of EGFP-expressing E coli HM615 across isolated human epithelium, in the presence and absence of plantain NSP.
Figure 5
Dietary emulsifier polysorbate-80 increases translocation of E coli across the intestinal epithelium. Polysorbate-80 increased translocation of Crohn's disease E coli across both (A) M-cells and (B) human follicle-associated epithelium (FAE). Similarly, polysorbate-80 enhanced translocation of E coli across (C) parent Caco2-cl1 cells and (D) human villus epithelium (VE). *, p<0.05; **, p<0.01; ANOVA (N=4–8). Crohn's E coli HM605 and EGFP-expressing HM615 were used for the in vitro and ex vivo epithelium translocation studies respectively.
Comment in
- Crohn's disease: soluble plant fibers may protect against E. coli translocation.
Franks I. Franks I. Nat Rev Gastroenterol Hepatol. 2010 Dec;7(12):650. doi: 10.1038/nrgastro.2010.180. Nat Rev Gastroenterol Hepatol. 2010. PMID: 21171217 No abstract available.
Similar articles
- Soluble plantain fibre blocks adhesion and M-cell translocation of intestinal pathogens.
Roberts CL, Keita AV, Parsons BN, Prorok-Hamon M, Knight P, Winstanley C, O' Kennedy N, Söderholm JD, Rhodes JM, Campbell BJ. Roberts CL, et al. J Nutr Biochem. 2013 Jan;24(1):97-103. doi: 10.1016/j.jnutbio.2012.02.013. Epub 2012 Jul 19. J Nutr Biochem. 2013. PMID: 22818716 Free PMC article. - Bile salts induce long polar fimbriae expression favouring Crohn's disease-associated adherent-invasive Escherichia coli interaction with Peyer's patches.
Chassaing B, Etienne-Mesmin L, Bonnet R, Darfeuille-Michaud A. Chassaing B, et al. Environ Microbiol. 2013 Feb;15(2):355-71. doi: 10.1111/j.1462-2920.2012.02824.x. Epub 2012 Jul 13. Environ Microbiol. 2013. PMID: 22789019 - Increased uptake of non-pathogenic E. coli via the follicle-associated epithelium in longstanding ileal Crohn's disease.
Keita AV, Salim SY, Jiang T, Yang PC, Franzén L, Söderkvist P, Magnusson KE, Söderholm JD. Keita AV, et al. J Pathol. 2008 Jun;215(2):135-44. doi: 10.1002/path.2337. J Pathol. 2008. PMID: 18348161 - Mucosal barrier, bacteria and inflammatory bowel disease: possibilities for therapy.
Merga Y, Campbell BJ, Rhodes JM. Merga Y, et al. Dig Dis. 2014;32(4):475-83. doi: 10.1159/000358156. Epub 2014 Jun 23. Dig Dis. 2014. PMID: 24969297 Review. - Barrier dysfunction and bacterial uptake in the follicle-associated epithelium of ileal Crohn's disease.
Keita AV, Söderholm JD. Keita AV, et al. Ann N Y Acad Sci. 2012 Jul;1258:125-34. doi: 10.1111/j.1749-6632.2012.06502.x. Ann N Y Acad Sci. 2012. PMID: 22731725 Review.
Cited by
- Current Nutritional Therapies in Inflammatory Bowel Disease: Improving Clinical Remission Rates and Sustainability of Long-Term Dietary Therapies.
Reznikov EA, Suskind DL. Reznikov EA, et al. Nutrients. 2023 Jan 28;15(3):668. doi: 10.3390/nu15030668. Nutrients. 2023. PMID: 36771373 Free PMC article. Review. - Impact of Diet on Risk of IBD.
Ananthakrishnan AN. Ananthakrishnan AN. Crohns Colitis 360. 2020 Jan 16;2(1):otz054. doi: 10.1093/crocol/otz054. eCollection 2020 Jan. Crohns Colitis 360. 2020. PMID: 36777955 Free PMC article. Review. - Eating for Two: Diet and the Microbiome in Ulcerative Colitis.
Lima SF, Longman RS. Lima SF, et al. J Crohns Colitis. 2022 Mar 14;16(3):341-342. doi: 10.1093/ecco-jcc/jjab181. J Crohns Colitis. 2022. PMID: 34718490 Free PMC article. No abstract available. - The Influence of Nutrients on Inflammatory Bowel Diseases.
Jarmakiewicz-Czaja S, Piątek D, Filip R. Jarmakiewicz-Czaja S, et al. J Nutr Metab. 2020 Feb 27;2020:2894169. doi: 10.1155/2020/2894169. eCollection 2020. J Nutr Metab. 2020. PMID: 32190385 Free PMC article. Review. - Environmental triggers for IBD.
O'Toole A, Korzenik J. O'Toole A, et al. Curr Gastroenterol Rep. 2014;16(7):396. doi: 10.1007/s11894-014-0396-y. Curr Gastroenterol Rep. 2014. PMID: 25048010 Review.
References
- Lees CW, Satsangi J. Genetics of inflammatory bowel disease: implications for disease pathogenesis and natural history. Expert Rev Gastroenterol Hepatol 2009;3:513–34 - PubMed
- Cottone M, Rosselli M, Orlando A, et al. Smoking habits and recurrence in Crohn's disease. Gastroenterology 1994;106:643–8 - PubMed
- Yao T, Matsui T, Hiwatashi N. Crohn's disease in Japan: diagnostic criteria and epidemiology. Dis Colon Rectum 2000;43:S85–93 - PubMed
- Akobeng AK, Thomas AG. Enteral nutrition for maintenance of remission in Crohn's disease. Cochrane Database Syst Rev 2007;(3):CD005984. - PubMed
- Knight P, Campbell BJ, Rhodes JM. Host-bacteria interaction in inflammatory bowel disease. Br Med Bull 2008;88:95–113 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical