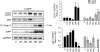Human-IAPP disrupts the autophagy/lysosomal pathway in pancreatic β-cells: protective role of p62-positive cytoplasmic inclusions - PubMed (original) (raw)
Human-IAPP disrupts the autophagy/lysosomal pathway in pancreatic β-cells: protective role of p62-positive cytoplasmic inclusions
J F Rivera et al. Cell Death Differ. 2011 Mar.
Abstract
In type II diabetes (T2DM), there is a deficit in β-cells, increased β-cell apoptosis and formation of intracellular membrane-permeant oligomers of islet amyloid polypeptide (IAPP). Human-IAPP (h-IAPP) is an amyloidogenic protein co-expressed with insulin by β-cells. IAPP expression is increased with obesity, the major risk factor for T2DM. In this study we report that increased expression of human-IAPP led to impaired autophagy, due at least in part to the disruption of lysosome-dependent degradation. This action of IAPP to alter lysosomal clearance in vivo depends on its propensity to form toxic oligomers and is independent of the confounding effect of hyperglycemia. We report that the scaffold protein p62 that delivers polyubiquitinated proteins to autophagy may have a protective role against human-IAPP-induced apoptosis, apparently by sequestrating protein targets for degradation. Finally, we found that inhibition of lysosomal degradation increases vulnerability of β-cells to h-IAPP-induced toxicity and, conversely, stimulation of autophagy protects β-cells from h-IAPP-induced apoptosis. Collectively, these data imply an important role for the p62/autophagy/lysosomal degradation system in protection against toxic oligomer-induced apoptosis.
Figures
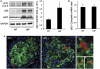
Figure 1
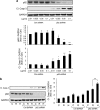
High expression of h-IAPP increases LC3-II and p62 protein levels and induces p62-positive cytoplasmic inclusions in HIP rats. (a) Protein levels of LC3, p62 and IAPP were assessed by western blot using islet protein lysates obtained from 4- to 6-month-old wild type (WT, _n_=6) and HIP rats (HIP, _n_=6). GAPDH was used as loading control. The graph represents the quantification of p62 protein levels. (b) Levels of p62 mRNA were evaluated by RT-qPCR in islets isolated from WT (_n_=3) and HIP rats (_n_=3). Data are expressed as mean±S.E.M.; *P<0.05. (c) p62 protein levels were assessed by immunofluorescence (p62, red; insulin, green; nuclei, blue) in pancreatic tissue from 4- to 6-month-old WT and HIP rats. High magnification of p62-positive structures found in HIP rats (i, ii and iii)
Figure 2
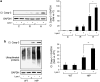
High expression of h-IAPP, but not r-IAPP, increases LC3-II and p62 protein levels and induces formation of p62-positive cytoplasmic inclusions in h-IAPP homozygous transgenic mice. (a) Protein levels of LC3 and p62 were assessed by western blot using islet protein lysates obtained from WT (WT, _n_=4), r-IAPP transgenic (r-TG, _n_=3) and h-IAPP transgenic mice (h-TG, _n_=4). GAPDH was used as loading control. The graph represents the quantification of LC3-II protein levels. (b) The graph represents the quantification of p62 protein levels. (c) Levels of p62 mRNA were evaluated by RT-qPCR in islets isolated from WT (_n_=3), r-TG (_n_=3) and h-TG mice (_n_=3). Data are expressed as mean±S.E.M.; *P<0.05. (d) p62 protein levels were assessed by immunofluorescence (p62, red; insulin, green; nuclei, blue) in pancreatic tissue from WT, r-TG and h-TG mice. High magnification of p62-positive structures found in h-TG mice (i, ii and iii)
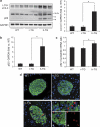
Figure 3
Interaction of obesity and h-IAPP expression, adaptive and maladaptive changes in autophagy. (a) Protein levels of LC3 and p62 were assessed by western blot using islet protein lysates obtained from lean non-transgenic (LNT, _n_=5), lean transgenic (LT, _n_=4), obese non-transgenic (ONT, _n_=5) and obese transgenic (OT, _n_=4–6) 10- to 12-week-old mice. Insulin and GAPDH were used as control. The graph represents the quantification of LC3-II protein levels. (b) The graph represents the quantification of p62 protein levels. (c) Levels of p62 mRNA were evaluated by RT-qPCR in islets isolated from LNT (_n_=5), LT (_n_=4), ONT (_n_=5) and OT mice (_n_=5). Data are expressed as mean±S.E.M.; *P<0.05; **P<0.01; ***P<0.001. (d) p62 protein levels were assessed by immunofluorescence (p62, red; insulin, green; nuclei, blue) in pancreatic tissue from LNT, LT, ONT and OT 10- to 12-week-old mice. (e) The graph represents the quantification of _β_-cells positive for p62 in each group (expressed in percentage). (f) The graph represents the quantification of _β_-cell area positive for p62 aggregates in each group (expressed in percentage). Data are expressed as mean±S.E.M.; *P<0.05; **P<0.01. The mathematical transformation ‘logarithm (value+1)' was applied before statistical analysis of LC3-II, p62 protein levels and _β_-cells positive for p62. (g) Fluorescence confocal images of p62 at magnification × 63 (p62, red; insulin, green) in pancreatic tissue from LNT, LT, ONT and OT 10- to 12-week-old mice
Figure 3
Interaction of obesity and h-IAPP expression, adaptive and maladaptive changes in autophagy. (a) Protein levels of LC3 and p62 were assessed by western blot using islet protein lysates obtained from lean non-transgenic (LNT, _n_=5), lean transgenic (LT, _n_=4), obese non-transgenic (ONT, _n_=5) and obese transgenic (OT, _n_=4–6) 10- to 12-week-old mice. Insulin and GAPDH were used as control. The graph represents the quantification of LC3-II protein levels. (b) The graph represents the quantification of p62 protein levels. (c) Levels of p62 mRNA were evaluated by RT-qPCR in islets isolated from LNT (_n_=5), LT (_n_=4), ONT (_n_=5) and OT mice (_n_=5). Data are expressed as mean±S.E.M.; *P<0.05; **P<0.01; ***P<0.001. (d) p62 protein levels were assessed by immunofluorescence (p62, red; insulin, green; nuclei, blue) in pancreatic tissue from LNT, LT, ONT and OT 10- to 12-week-old mice. (e) The graph represents the quantification of _β_-cells positive for p62 in each group (expressed in percentage). (f) The graph represents the quantification of _β_-cell area positive for p62 aggregates in each group (expressed in percentage). Data are expressed as mean±S.E.M.; *P<0.05; **P<0.01. The mathematical transformation ‘logarithm (value+1)' was applied before statistical analysis of LC3-II, p62 protein levels and _β_-cells positive for p62. (g) Fluorescence confocal images of p62 at magnification × 63 (p62, red; insulin, green) in pancreatic tissue from LNT, LT, ONT and OT 10- to 12-week-old mice
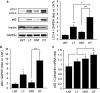
Figure 4
High expression of h-IAPP in INS 832/13 cells increases LC3-II and affects p62 protein levels. INS 832/13 cells were transduced at 400 MOI with r-IAPP or h-IAPP adenoviruses and cell lysates were collected at different time points as indicated (time 0, non-transduced cells). Protein levels of LC3, p62, cleaved caspase-3 (Cl. Casp-3) and IAPP were assessed by western blot. GAPDH was used as loading control. The graphs represent the quantification of LC3-II and p62 protein levels (_n_=4). Data are expressed as mean±S.E.M.; *P<0.05; **P<0.01; ***P<0.001, significant differences versus non-transduced cells (Ctrl)
Figure 5
Downregulation of p62 by shRNA lentivirus induces apoptosis in INS 832/13 cells and exacerbates h-IAPP-induced _β_-cell apoptosis. (a) INS 832/13 cells were transduced for 72 h with control shRNA or p62 shRNA lentivirus at increasing concentrations. Levels of p62 and cleaved caspase-3 (Cl. Casp-3) were analyzed by western blot. GAPDH was used as loading control. The graphs represent the quantification of p62 protein levels and the cleaved form of caspase-3 (_n_=3). Data are expressed as mean±S.E.M.; *P<0.05; **P<0.01; ***P<0.001, significant differences versus Ctrl shRNA-transduced cells (at the corresponding concentration). (b) INS 832/13 cells were transduced for 48 h with control shRNA or p62 shRNA lentivirus (0.1 _μ_g/ml) and transduced at 400 MOI with r-IAPP (R) or h-IAPP (H) adenoviruses for the last 27 h (C, non-transduced cells). Levels of cleaved caspase-3 and p62 were analyzed by western blot. GAPDH was used as loading control. The graph represents the quantification of the cleaved form of caspase-3 (_n_=4). Data are expressed as mean±S.E.M.; ***P<0.001
Figure 6
Overexpression of p62 in INS 832/13 cells reduces h-IAPP-induced _β_-cell apoptosis. (a) INS 832/13 cells were transfected with GFP plasmid or p62-GFP plasmid (0.1_μ_g) for 24 h and observed under fluorescence microscope (p62, green; nuclei, blue). (b) INS 832/13 cells were transduced at 400 MOI with r-IAPP (R) or h-IAPP (H) adenoviruses for 36 h (C, non-transduced cells). Cells were transfected with GFP plasmid or p62-GFP plasmid (0.1 _μ_g) for the last 22 h. Levels of p62 and cleaved caspase-3 (Cl. Casp-3) were analyzed by western blot. GAPDH was used as loading control. The graph represents the quantification of the cleaved form of caspase-3 (_n_=4). Data are expressed as mean±S.E.M.; *P<0.05
Figure 7
Inhibition of lysosomal degradation exacerbates h-IAPP-induced _β_-cell apoptosis in INS 832/13 cells and rat islets. (a) INS 832/13 cells were transduced at 400 MOI with r-IAPP (R) or h-IAPP (H) adenoviruses for 36 h (C, non-transduced cells) and treated with lysosomal inhibitors (Lyso I) (E-64-d, 10 _μ_g/ml and pepstatin A, 10 _μ_g/ml) for the last 24 h. Levels of cleaved caspase-3 (Cl. Casp-3) were analyzed by western blot. GAPDH was used as loading control. The graph represents the quantification of the cleaved form of caspase-3 (_n_=4). Data are expressed as mean±S.E.M.; *P<0.05. (b) Isolated islets obtained from wild-type (WT: _n_=3) and HIP rats (HIP: _n_=5) were treated with lysosomal inhibitors (E-64-d, 10 _μ_g/ml and pepstatin A, 10 _μ_g/ml) for 24 h. Levels of cleaved caspase-3 and ubiquitin were analyzed by western blot. GAPDH was used as loading control. The graph represents the quantification of the cleaved form of caspase-3. Data are expressed as mean±S.E.M.; **P<0.01
Figure 8
Enhanced autophagy by treatment with rapamycin decreases h-IAPP-induced apoptosis in INS 832/13 cells. INS 832/13 cells were transduced at 400 MOI with r-IAPP (R) or h-IAPP (H) adenoviruses for 48 h (C, non-transduced cells) and treated with rapamycin (Rapa, 10 n) for the last 40 h. Levels of P-mTOR and cleaved caspase-3 (Cl. Casp-3) were analyzed by western blot. GAPDH was used as loading control. The graph represents the quantification of the cleaved form of caspase-3 (_n_=4). Data are expressed as mean±S.E.M.; **P<0.01; ***P<0.001
Similar articles
- Autophagy defends pancreatic β cells from human islet amyloid polypeptide-induced toxicity.
Rivera JF, Costes S, Gurlo T, Glabe CG, Butler PC. Rivera JF, et al. J Clin Invest. 2014 Aug;124(8):3489-500. doi: 10.1172/JCI71981. Epub 2014 Jul 18. J Clin Invest. 2014. PMID: 25036708 Free PMC article. - UCHL1 deficiency exacerbates human islet amyloid polypeptide toxicity in β-cells: evidence of interplay between the ubiquitin/proteasome system and autophagy.
Costes S, Gurlo T, Rivera JF, Butler PC. Costes S, et al. Autophagy. 2014 Jun;10(6):1004-14. doi: 10.4161/auto.28478. Autophagy. 2014. PMID: 24879150 Free PMC article. - β-cell dysfunctional ERAD/ubiquitin/proteasome system in type 2 diabetes mediated by islet amyloid polypeptide-induced UCH-L1 deficiency.
Costes S, Huang CJ, Gurlo T, Daval M, Matveyenko AV, Rizza RA, Butler AE, Butler PC. Costes S, et al. Diabetes. 2011 Jan;60(1):227-38. doi: 10.2337/db10-0522. Epub 2010 Oct 27. Diabetes. 2011. PMID: 20980462 Free PMC article. - Causative factors for formation of toxic islet amyloid polypeptide oligomer in type 2 diabetes mellitus.
Jeong HR, An SS. Jeong HR, et al. Clin Interv Aging. 2015 Nov 19;10:1873-9. doi: 10.2147/CIA.S95297. eCollection 2015. Clin Interv Aging. 2015. PMID: 26604727 Free PMC article. Review. - Islet amyloid polypeptide (IAPP) transgenic rodents as models for type 2 diabetes.
Matveyenko AV, Butler PC. Matveyenko AV, et al. ILAR J. 2006;47(3):225-33. doi: 10.1093/ilar.47.3.225. ILAR J. 2006. PMID: 16804197 Review.
Cited by
- A Selective Look at Autophagy in Pancreatic β-Cells.
Pearson GL, Gingerich MA, Walker EM, Biden TJ, Soleimanpour SA. Pearson GL, et al. Diabetes. 2021 Jun;70(6):1229-1241. doi: 10.2337/dbi20-0014. Epub 2021 May 20. Diabetes. 2021. PMID: 34016598 Free PMC article. Review. - Lysosomal degradation of newly formed insulin granules contributes to β cell failure in diabetes.
Pasquier A, Vivot K, Erbs E, Spiegelhalter C, Zhang Z, Aubert V, Liu Z, Senkara M, Maillard E, Pinget M, Kerr-Conte J, Pattou F, Marciniak G, Ganzhorn A, Ronchi P, Schieber NL, Schwab Y, Saftig P, Goginashvili A, Ricci R. Pasquier A, et al. Nat Commun. 2019 Jul 25;10(1):3312. doi: 10.1038/s41467-019-11170-4. Nat Commun. 2019. PMID: 31346174 Free PMC article. - CHOP Contributes to, But Is Not the Only Mediator of, IAPP Induced β-Cell Apoptosis.
Gurlo T, Rivera JF, Butler AE, Cory M, Hoang J, Costes S, Butler PC. Gurlo T, et al. Mol Endocrinol. 2016 Apr;30(4):446-54. doi: 10.1210/me.2015-1255. Epub 2016 Feb 22. Mol Endocrinol. 2016. PMID: 26900721 Free PMC article. - Big versus small: The impact of aggregate size in disease.
Hnath B, Chen J, Reynolds J, Choi E, Wang J, Zhang D, Sha CM, Dokholyan NV. Hnath B, et al. Protein Sci. 2023 Jul;32(7):e4686. doi: 10.1002/pro.4686. Protein Sci. 2023. PMID: 37243896 Free PMC article. Review. - Proteasome regulates turnover of toxic human amylin in pancreatic cells.
Singh S, Trikha S, Sarkar A, Jeremic AM. Singh S, et al. Biochem J. 2016 Sep 1;473(17):2655-70. doi: 10.1042/BCJ20160026. Epub 2016 Jun 23. Biochem J. 2016. PMID: 27340132 Free PMC article.
References
- Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes. 2003;52:102–110. - PubMed
- Kloppel G, Lohr M, Habich K, Oberholzer M, Heitz PU. Islet pathology and the pathogenesis of type 1 and type 2 diabetes mellitus revisited. Surv Synth Pathol Res. 1985;4:110–125. - PubMed
- Huang CJ, Lin CY, Haataja L, Gurlo T, Butler AE, Rizza RA, et al. High expression rates of human islet amyloid polypeptide induce endoplasmic reticulum stress-mediated beta-cell apoptosis, a characteristic of humans with type 2 but not type 1 diabetes. Diabetes. 2007;56:2016–2027. - PubMed
- Marchetti P, Bugliani M, Lupi R, Marselli L, Masini M, Boggi U, et al. The endoplasmic reticulum in pancreatic beta cells of type 2 diabetes patients. Diabetologia. 2007;50:2486–2494. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials