Evidence that weakened centromere cohesion is a leading cause of age-related aneuploidy in oocytes - PubMed (original) (raw)
Evidence that weakened centromere cohesion is a leading cause of age-related aneuploidy in oocytes
Teresa Chiang et al. Curr Biol. 2010.
Abstract
Aneuploidy arising early in development is the leading genetic cause of birth defects and developmental disabilities in humans. Most errors in chromosome number originate from the egg, and maternal age is well established as the key risk factor. Although the importance of this problem for reproductive health is widely recognized, the underlying molecular basis for age-related aneuploidy in female meiosis is unknown. Here we show that weakened chromosome cohesion is a leading cause of aneuploidy in oocytes in a natural aging mouse model. We find that sister kinetochores are farther apart at both metaphase I and II, indicating reduced centromere cohesion. Moreover, levels of the meiotic cohesin protein REC8 are severely reduced on chromosomes in oocytes from old mice. To test whether cohesion defects lead to the observed aneuploidies, we monitored chromosome segregation dynamics at anaphase I in live oocytes and counted chromosomes in the resulting metaphase II eggs. About 90% of age-related aneuploidies are best explained by weakened centromere cohesion. Together, these results demonstrate that the maternal age-associated increase in aneuploidy is often due to a failure to effectively replace cohesin proteins that are lost from chromosomes during aging.
Copyright © 2010 Elsevier Ltd. All rights reserved.
Figures
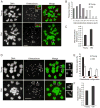
Figure 1. Sister kinetochores are farther apart in old oocytes both at MII and MI
A-C, Oocytes from young (6–14 weeks of age) or old (16–19 months of age) mice were matured in vitro, treated with monastrol to disperse the chromosomes at MII, then fixed and stained for DNA (Sytox, green) and kinetochores (CREST, red). Representative images (A) show increased distance between sister kinetochores, with insets magnified to show sister kinetochore pairs. Inter-kinetochore distances were measured for all sister kinetochore pairs in an oocyte and averaged over each oocyte. The populations of young (n=24) and old (n=17) oocytes are represented by histograms (B) and by the mean (±sem) for each group (C). D-F, Oocytes were treated with monastrol at MI, then fixed and stained as above. Insets (D) show increased separation of sister kinetochores; each box includes all four kinetochores of a bivalent. Sister kinetochore configurations in MI were classified as indistinguishable, overlapping, or distinct (E, left to right). Outer kinetochore distances were measured from the outer edges of sister kinetochore pairs for young and old oocytes (n=200 kinetochore pairs from 10 oocytes in each group) (F). Images (A, D) are maximal intensity projections of confocal z-series, scale bars 5 μm. See also Figure S1.
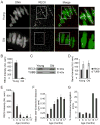
Figure 2. REC8 protein levels on chromosomes are severely reduced in old oocytes while total REC8 is constant
A, B, Chromosome-associated REC8 was detected by immunocytochemistry in young and old oocytes at MI. Representative images (A) show DNA (Sytox, green) and REC8 (red), scale bar 5 μm. Similar results were obtained with multiple young (n=11) and old (n=9) oocytes, and the REC8 fluorescence intensity was quantified (B). C, D, Total REC8 levels in young and old oocytes at MI (n=80 oocytes in each lane) were determined by Western blot, with TUBB as a loading control. A representative blot is shown; similar results were obtained in two independent experiments, and band intensities were quantified (D). E, Levels of chromosome-associated REC8 were measured as in (A) with oocytes from mice at different ages (in months): 3 (n=21), 6 (n=21), 9 (n=19), 12 (n=21), 15 (n=7), 17 (n=3). F, G, MII eggs from mice at various ages were analyzed to determine inter-kinetochore distances (F) as in Fig. 1A-C, and the percent of eggs with chromosome segregation errors, including aneuploidies and separated sister chromatids, was determined (G). Mice were used at the following different ages (in months): 3 (n=39), 6 (n>28), 9 (n=20), 12 (n>24), 15 (n>14), 17 (n=16). See also Figure S2.
Figure 3. Proposed outcomes of weakened centromere cohesion in MI
Schematics show the predicted outcomes of normal (A) or weakened (B-E) centromere cohesion in MI. Red boxes follow the progression of a sister kinetochore pair. Cohesion status and sister kinetochore orientation on the MI spindle determine chromosome segregation dynamics in Anaphase I and the chromosome content of the MII egg. For prediction (E), sister kinetochore separation is shown at MI but could alternatively arise at a later time. Note that mouse chromosomes are telocentric.
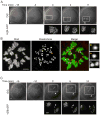
Figure 4. Analysis of Anaphase I chromosome segregation dynamics and MII chromosome numbers in old oocytes
A, An old oocyte was micro-injected with H2b-gfp cRNA, then matured in vitro and imaged live: initially by DIC to determine the time of anaphase onset (t=0) and subsequently by fluorescence to detect lagging chromosomes in Anaphase I. GFP images are magnified from white boxes in the DIC images. No lagging chromosomes are detected in this example. B, The MII egg resulting from the oocyte shown in (A) was fixed and stained for DNA (Sytox, green) and kinetochores (CREST, red) to count chromosomes. The egg is euploid with 19 paired sister kinetochores (numbered 1–19) and two unpaired kinetochores (yellow and orange asterisks). Insets show the two unpaired kinetochores and an intact sister kinetochore pair. C, Another old oocyte was imaged live in Meiosis I as in (A). In this example a lagging chromosome (arrow) eventually segregates to the polar body. The final kinetochore count in the MII egg is 39, indicating loss of a single chromatid. Arrowheads mark the polar bodies; GFP images are maximal intensity projections of confocal z-series; scale bars 5 μm.
Similar articles
- The horse as a natural model to study reproductive aging-induced aneuploidy and weakened centromeric cohesion in oocytes.
Rizzo M, du Preez N, Ducheyne KD, Deelen C, Beitsma MM, Stout TAE, de Ruijter-Villani M. Rizzo M, et al. Aging (Albany NY). 2020 Nov 2;12(21):22220-22232. doi: 10.18632/aging.104159. Epub 2020 Nov 2. Aging (Albany NY). 2020. PMID: 33139583 Free PMC article. - Manipulating Cohesin Levels in Live Mouse Oocytes.
Szydłowska A, Ladstätter S, Tachibana K. Szydłowska A, et al. Methods Mol Biol. 2018;1818:113-128. doi: 10.1007/978-1-4939-8603-3_12. Methods Mol Biol. 2018. PMID: 29961260 - Age-dependent loss of cohesion protection in human oocytes.
Mihalas BP, Pieper GH, Aboelenain M, Munro L, Srsen V, Currie CE, Kelly DA, Hartshorne GM, Telfer EE, McAinsh AD, Anderson RA, Marston AL. Mihalas BP, et al. Curr Biol. 2024 Jan 8;34(1):117-131.e5. doi: 10.1016/j.cub.2023.11.061. Epub 2023 Dec 21. Curr Biol. 2024. PMID: 38134935 Free PMC article. - Age-Related Loss of Cohesion: Causes and Effects.
Cheng JM, Liu YX. Cheng JM, et al. Int J Mol Sci. 2017 Jul 22;18(7):1578. doi: 10.3390/ijms18071578. Int J Mol Sci. 2017. PMID: 28737671 Free PMC article. Review. - Chromosome Segregation in the Oocyte: What Goes Wrong during Aging.
Wasielak-Politowska M, Kordowitzki P. Wasielak-Politowska M, et al. Int J Mol Sci. 2022 Mar 7;23(5):2880. doi: 10.3390/ijms23052880. Int J Mol Sci. 2022. PMID: 35270022 Free PMC article. Review.
Cited by
- Subcortical maternal complex (SCMC) expression during folliculogenesis is affected by oocyte donor age in sheep.
Bebbere D, Abazari-Kia A, Nieddu S, Melis Murgia B, Albertini DF, Ledda S. Bebbere D, et al. J Assist Reprod Genet. 2020 Sep;37(9):2259-2271. doi: 10.1007/s10815-020-01871-x. Epub 2020 Jul 1. J Assist Reprod Genet. 2020. PMID: 32613414 Free PMC article. - Effects of post-ovulatory aging on centromeric cohesin protection in murine MII oocytes.
Shimoi G, Wakabayashi R, Ishikawa R, Kameyama Y. Shimoi G, et al. Reprod Med Biol. 2021 Dec 13;21(1):10.1002/rmb2.12433. doi: 10.1002/rmb2.12433. eCollection 2022 Jan-Dec. Reprod Med Biol. 2021. PMID: 35386382 Free PMC article. - Effects of aneuploidy on cell behaviour and function.
Li R, Zhu J. Li R, et al. Nat Rev Mol Cell Biol. 2022 Apr;23(4):250-265. doi: 10.1038/s41580-021-00436-9. Epub 2022 Jan 5. Nat Rev Mol Cell Biol. 2022. PMID: 34987171 Review. - Maternal Smc3 protects the integrity of the zygotic genome through DNA replication and mitosis.
Yueh WT, Singh VP, Gerton JL. Yueh WT, et al. Development. 2021 Dec 15;148(24):dev199800. doi: 10.1242/dev.199800. Epub 2021 Dec 22. Development. 2021. PMID: 34935904 Free PMC article. - Sex Differences in the Recombination Landscape.
Sardell JM, Kirkpatrick M. Sardell JM, et al. Am Nat. 2020 Feb;195(2):361-379. doi: 10.1086/704943. Epub 2019 Dec 9. Am Nat. 2020. PMID: 32017625 Free PMC article.
References
- Penrose LS. The relative effects of paternal and maternal age in mongolism. Journal of genetics. 1933;88:9–14. - PubMed
- Hassold T, Hunt P. To err (meiotically) is human: the genesis of human aneuploidy. Nat Rev Genet. 2001;2:280–291. - PubMed
- Hassold T, Hall H, Hunt P. The origin of human aneuploidy: where we have been, where we are going. Human molecular genetics. 2007;16(Spec No 2):R203–208. - PubMed
- Jones KT. Meiosis in oocytes: predisposition to aneuploidy and its increased incidence with age. Human reproduction update. 2008;14:143–158. - PubMed
- Koehler KE, Schrump SE, Cherry JP, Hassold TJ, Hunt PA. Near-human aneuploidy levels in female mice with homeologous chromosomes. Curr Biol. 2006;16:R579–580. - PubMed
Publication types
MeSH terms
Grants and funding
- T32 HD007305/HD/NICHD NIH HHS/United States
- HD055822/HD/NICHD NIH HHS/United States
- HD 058730/HD/NICHD NIH HHS/United States
- R01 HD058730/HD/NICHD NIH HHS/United States
- T32 HD 007305/HD/NICHD NIH HHS/United States
- F32 HD055822/HD/NICHD NIH HHS/United States
- R00 HD061657/HD/NICHD NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials