CSF Aβ(42) and tau in Parkinson's disease with cognitive impairment - PubMed (original) (raw)
. 2010 Nov 15;25(15):2682-5.
doi: 10.1002/mds.23287.
Min Shi, Joseph F Quinn, Elaine R Peskind, Suzanne Craft, Carmen Ginghina, Kathryn A Chung, Hojoong Kim, Douglas R Galasko, Joseph Jankovic, Cyrus P Zabetian, James B Leverenz, Jing Zhang
Affiliations
- PMID: 20818673
- PMCID: PMC2978754
- DOI: 10.1002/mds.23287
CSF Aβ(42) and tau in Parkinson's disease with cognitive impairment
Thomas J Montine et al. Mov Disord. 2010.
Abstract
We tested the hypothesis that the CSF biomarker signature associated with Alzheimer's disease (AD) is present in a subset of individuals with Parkinson's disease and Dementia (PD-D) or with PD and Cognitive Impairment, Not Dementia (PD-CIND). We quantified CSF Aβ(42), total tau (T-tau), and phospho-tau (P181-tau) using commercially available kits. Samples were from 345 individuals in seven groups (n): Controls ≤50 years (35), Controls >50 years (115), amnestic Mild Cognitive Impairment (aMCI) (24), AD (49), PD (49), PD-CIND (62), and PD-D (11). We observed expected changes in AD or aMCI compared with age-matched or younger controls. CSF Aβ(42) was reduced in PD-CIND (P < 0.05) and PD-D (P < 0.01), whereas average CSF T-tau and P181-tau were unchanged or decreased. One-third of PD-CIND and one-half of PD-D patients had the biomarker signature of AD. Abnormal metabolism of Aβ(42) may be a common feature of PD-CIND and PD-D.
© 2010 Movement Disorder Society.
Figures
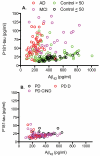
Figure 1. Scatter plots of CSF Aβ42 and P181-tau concentrations
Individuals’ CSF Aβ42 and P181-tau concentrations for (A) both Control groups, subjects with amnestic Mild Cognitive Impairment (MCI), and patients with Alzheimer’s Disease (AD), and for (B) patients with Parkinson’s disease (PD) without cognitive impairment (CI), with CI but Not Dementia (PD-CIND), or dementia (PD-D).
Similar articles
- CSF amyloid-beta and tau proteins, and cognitive performance, in early and untreated Parkinson's disease: the Norwegian ParkWest study.
Alves G, Brønnick K, Aarsland D, Blennow K, Zetterberg H, Ballard C, Kurz MW, Andreasson U, Tysnes OB, Larsen JP, Mulugeta E. Alves G, et al. J Neurol Neurosurg Psychiatry. 2010 Oct;81(10):1080-6. doi: 10.1136/jnnp.2009.199950. Epub 2010 Jun 14. J Neurol Neurosurg Psychiatry. 2010. PMID: 20547614 - Amyloid-β and α-synuclein cerebrospinal fluid biomarkers and cognition in early Parkinson's disease.
Stav AL, Aarsland D, Johansen KK, Hessen E, Auning E, Fladby T. Stav AL, et al. Parkinsonism Relat Disord. 2015 Jul;21(7):758-64. doi: 10.1016/j.parkreldis.2015.04.027. Epub 2015 May 2. Parkinsonism Relat Disord. 2015. PMID: 25971633 - Cerebrospinal Fluid Alzheimer's Disease Biomarkers Across the Spectrum of Lewy Body Diseases: Results from a Large Multicenter Cohort.
van Steenoven I, Aarsland D, Weintraub D, Londos E, Blanc F, van der Flier WM, Teunissen CE, Mollenhauer B, Fladby T, Kramberger MG, Bonanni L, Lemstra AW; European DLB consortium. van Steenoven I, et al. J Alzheimers Dis. 2016 Aug 18;54(1):287-95. doi: 10.3233/JAD-160322. J Alzheimers Dis. 2016. PMID: 27567832 Free PMC article. - Biological confounders for the values of cerebrospinal fluid proteins in Parkinson's disease and related disorders.
Mollenhauer B, Parnetti L, Rektorova I, Kramberger MG, Pikkarainen M, Schulz-Schaeffer WJ, Aarsland D, Svenningsson P, Farotti L, Verbeek MM, Schlossmacher MG. Mollenhauer B, et al. J Neurochem. 2016 Oct;139 Suppl 1:290-317. doi: 10.1111/jnc.13390. Epub 2016 Feb 10. J Neurochem. 2016. PMID: 26452984 Review.
Cited by
- Cerebrospinal fluid Aβ42 levels and APP processing pathway genes in Parkinson's disease.
Bekris LM, Tsuang DW, Peskind ER, Yu CE, Montine TJ, Zhang J, Zabetian CP, Leverenz JB. Bekris LM, et al. Mov Disord. 2015 Jun;30(7):936-44. doi: 10.1002/mds.26172. Epub 2015 Mar 24. Mov Disord. 2015. PMID: 25808939 Free PMC article. - A First Tetraplex Assay for the Simultaneous Quantification of Total α-Synuclein, Tau, β-Amyloid42 and DJ-1 in Human Cerebrospinal Fluid.
Kruse N, Schlossmacher MG, Schulz-Schaeffer WJ, Vanmechelen E, Vanderstichele H, El-Agnaf OM, Mollenhauer B. Kruse N, et al. PLoS One. 2016 Apr 26;11(4):e0153564. doi: 10.1371/journal.pone.0153564. eCollection 2016. PLoS One. 2016. PMID: 27116005 Free PMC article. - Magnetic Resonance Imaging Studies of Neurodegenerative Disease: From Methods to Translational Research.
Huang P, Zhang M. Huang P, et al. Neurosci Bull. 2023 Jan;39(1):99-112. doi: 10.1007/s12264-022-00905-x. Epub 2022 Jun 30. Neurosci Bull. 2023. PMID: 35771383 Free PMC article. Review. - APOE genotype regulates pathology and disease progression in synucleinopathy.
Davis AA, Inman CE, Wargel ZM, Dube U, Freeberg BM, Galluppi A, Haines JN, Dhavale DD, Miller R, Choudhury FA, Sullivan PM, Cruchaga C, Perlmutter JS, Ulrich JD, Benitez BA, Kotzbauer PT, Holtzman DM. Davis AA, et al. Sci Transl Med. 2020 Feb 5;12(529):eaay3069. doi: 10.1126/scitranslmed.aay3069. Sci Transl Med. 2020. PMID: 32024799 Free PMC article. - Narrative Organization Deficit in Lewy Body Disorders Is Related to Alzheimer Pathology.
Grossman M, Irwin DJ, Jester C, Halpin A, Ash S, Rascovsky K, Weintraub D, McMillan CT. Grossman M, et al. Front Neurosci. 2017 Feb 8;11:53. doi: 10.3389/fnins.2017.00053. eCollection 2017. Front Neurosci. 2017. PMID: 28228714 Free PMC article.
References
- Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256:183–194. - PubMed
- Mattsson N, Zetterberg H, Hansson O, et al. CSF biomarkers and incipient Alzheimer disease in patients with mild cognitive impairment. JAMA. 2009;302:385–393. - PubMed
- Li G, Sokal I, Quinn JF, et al. CSF tau/Abeta42 ratio for increased risk of mild cognitive impairment: a follow-up study. Neurology. 2007;69:631–639. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- ES004696/ES/NIEHS NIH HHS/United States
- NS057567/NS/NINDS NIH HHS/United States
- R21 NS060252/NS/NINDS NIH HHS/United States
- P30 AG008017/AG/NIA NIH HHS/United States
- P50 NS062684/NS/NINDS NIH HHS/United States
- R01 AG033398/AG/NIA NIH HHS/United States
- AG05136/AG/NIA NIH HHS/United States
- NS060252/NS/NINDS NIH HHS/United States
- AG08017/AG/NIA NIH HHS/United States
- AG033398/AG/NIA NIH HHS/United States
- R01 NS057567/NS/NINDS NIH HHS/United States
- NS062684/NS/NINDS NIH HHS/United States
- R01 AG025327/AG/NIA NIH HHS/United States
- P42 ES004696/ES/NIEHS NIH HHS/United States
- P50 AG008671/AG/NIA NIH HHS/United States
- P50 AG005136/AG/NIA NIH HHS/United States
- AG025327/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical