Mature natural killer cells reset their responsiveness when exposed to an altered MHC environment - PubMed (original) (raw)
Mature natural killer cells reset their responsiveness when exposed to an altered MHC environment
Nathalie T Joncker et al. J Exp Med. 2010.
Abstract
Some mature natural killer (NK) cells cannot be inhibited by major histocompatibility complex (MHC) I molecules, either because they lack corresponding inhibitory receptors or because the host lacks the corresponding MHC I ligands for the receptors. Such NK cells nevertheless remain self-tolerant and exhibit a generalized hyporesponsiveness to stimulation through activating receptors. To address whether NK cell responsiveness is set only during the NK cell differentiation process, we transferred mature NK cells from wild-type (WT) to MHC I-deficient hosts or vice versa. Remarkably, mature responsive NK cells from WT mice became hyporesponsive after transfer to MHC I-deficient mice, whereas mature hyporesponsive NK cells from MHC I-deficient mice became responsive after transfer to WT mice. Altered responsiveness was evident among mature NK cells that had not divided in the recipient animals, indicating that the cells were mature before transfer and that alterations in activity did not require cell division. Furthermore, the percentages of NK cells expressing KLRG1, CD11b, CD27, and Ly49 receptors specific for H-2(b) were not markedly altered after transfer. Thus, the functional activity of mature NK cells can be reset when the cells are exposed to a changed MHC environment. These findings have important implications for how NK cell functions may be curtailed or enhanced in the context of disease.
Figures
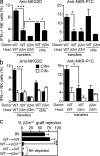
Figure 1.
Responsive NK cells reset their functional potential downward in the absence of MHC I contact. Responses of WT NK cells after transfer to WT or β2m-deficient hosts. (a and b) 10 d after transfer, splenic NK cells were stimulated for 5 h with 5 µg/ml of plate-bound NKG2D or 50 µg/ml NKR-P1C antibody as indicated. The percentage of IFN-γ–producing NK cells among gated donor NK cells (a) or gated donor NK cells expressing one or more of Ly49C, Ly49I, and/or CD94/NKG2A (CIN+) or lacking those three receptors (CIN−; b) was determined by flow cytometry. Responses of NK cells from unmanipulated WT and β2m−/− mice are shown for comparison. Experiments were repeated five to six times with n = 3–5 each for transfer groups. For relevant comparisons, statistical significance was determined with a two-tailed unpaired (a) or paired (b) Student’s t test (*, P < 0.05; ***, P < 0.0005). ctrls, controls. (c) At day 12 after transfer, groups of adoptive transfer recipients (n = 3–4) received grafts of CFSEhigh-labeled β2m−/− spleen cells mixed with an equal number of CFSElow-labeled WT spleen cells. Rejection of β2m−/− target cells was determined by flow cytometry of spleen cells 18 h later. Some groups of transfer recipients were pretreated i.p. twice (on days −2 and −1 relative to the time of engraftment) with 200 µg PK136 (NK1.1) antibody to deplete NK cells, as indicated. The experiment was repeated three times with n = 3–6 mice for each (***, P = 0.0002). Data represent means ± SEM.
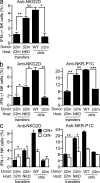
Figure 2.
Hyporesponsive NK cells reset their functional potential upwards when exposed to MHC I–expressing cells. (a–c) Responses of β2m−/− NK cells after transfer to MHC I–expressing NK cell–deficient (NKD) or β2m-deficient hosts. Stimulations and assays were performed as in Fig. 1. The percentage of IFN-γ–producing NK cells among gated donor NK cells (a and b) or gated donor CIN+ and CIN− NK cells (c) was determined by flow cytometry. Recipients were either left untreated (a) or treated with Poly I:C (b and c) 36 h before in vitro stimulation to augment the responses. Experiments were repeated four times with n = 3–6 mice each for transfer groups. For relevant comparisons, statistical significance was determined with a two-tailed unpaired (a and b) or paired (c) Student’s t test (*, P < 0.05; **, P < 0.005; ***, P < 0.0005). Data represent means ± SEM. ctrls, controls.
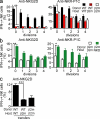
Figure 3.
Functional resetting occurs even in NK cells that do not divide after transfer. (a) CFSE-labeled WT splenocytes were transferred to irradiated WT or β2m-deficient hosts. (b) CFSE-labeled β2m−/− splenocytes were transferred to irradiated β2m−/− or MHC I–expressing NK cell–deficient (NKD) hosts. 10 d after transfer, NK cells were stimulated and assayed as in Fig. 1, except that the donor cells were gated based on CFSE level, distinguishing cells that had divided 0, 1, 2, 3, or 4 times after initial labeling. Experiments were repeated two to four times with n = 3–6 mice for each. (c) WT splenocytes were transferred to unirradiated, CD8a- and CD4-depleted WT, or β2m-deficient hosts. 9 d after transfer, NK cells were stimulated and assayed as in Fig. 1. The experiment was performed twice with n = 3–5 mice. For some comparisons, statistical significance was determined with a two-tailed unpaired Student’s t test (*, P < 0.05; **, P < 0.005; ***, P < 0.0005). Data represent means ± SEM.
Figure 4.
Phenotypic changes associated with resetting. (a) 10 d after transfer of CFSE-labeled splenocytes, the frequencies of CIN+ and CIN− NK cells were determined among gated donor NK cells that remained undivided after transfer by staining with Ly49C, Ly49I, and NKG2A antibodies. (b) IFN-γ response of KLRG1high and KLRG1low WT NK cells to stimulation with 5 µg/ml plate-bound NKG2D antibody. (c) Percentages of KLRG1high cells among undivided donor NK cells at day 10 after transfer. (d) IFN-γ response of CD27− CD11b−, CD27+ CD11b−, CD27+ CD11b+, and CD27− CD11b+ NK cell subsets from WT mice upon stimulation with 5 µg/ml anti-NKG2D. (e) Distribution of undivided donor NK cells among the CD27/CD11b-defined subsets at day 10 after transfer. Asterisks indicate a statistically significant difference as calculated by two-tailed paired (b) or unpaired (c) Student’s t test (*, P < 0.05; ***, P < 0.0005). Experiments were performed two to four times with n = 3–6 mice, except one repeat of panel d where n = 2 mice. Data represent means ± SEM.
Figure 5.
The responsiveness of developing NK cells is set depending on the MHC environment in which they mature. (a) Experimental scheme for producing mixed fetal liver cell chimeras and testing rejection of MHC I–deficient cells. WT (+), β2m−/− (−), or a 1:1 mix of WT and β2m−/− (mix) fetal liver cells was used to reconstitute lethally irradiated and NK-depleted hosts. (b) After 9–17 wk of reconstitution, chimeras were reirradiated or not before testing whether the chimeras rejected β2m−/− spleen cells or bone marrow cells, as indicated. Unmanipulated B6 and β2m−/− mice were tested in parallel as controls for rejection and tolerance, respectively. The rejection assay was performed by injecting CFSEhigh-labeled β2m−/− cells (spleen or bone marrow) mixed with an equal number of CFSElow-labeled WT spleen cells as an internal control. Rejection of β2m−/− spleen cells was determined by flow cytometry of spleen cells 18 h later. Some groups of chimeras were pretreated i.p. twice (on days −2 and −1) with 200 µg PK136 (NK1.1) antibody (NK depleted), as indicated. Data represent means ± SEM (n = 2–5 mice). The experiment was performed three times with spleen cell targets and once with bone marrow cell targets. nd, not done.
Similar articles
- MHC class I-deficient natural killer cells acquire a licensed phenotype after transfer into an MHC class I-sufficient environment.
Elliott JM, Wahle JA, Yokoyama WM. Elliott JM, et al. J Exp Med. 2010 Sep 27;207(10):2073-9. doi: 10.1084/jem.20100986. Epub 2010 Sep 6. J Exp Med. 2010. PMID: 20819924 Free PMC article. - Impaired natural killer cell self-education and "missing-self" responses in Ly49-deficient mice.
Bélanger S, Tu MM, Rahim MM, Mahmoud AB, Patel R, Tai LH, Troke AD, Wilhelm BT, Landry JR, Zhu Q, Tung KS, Raulet DH, Makrigiannis AP. Bélanger S, et al. Blood. 2012 Jul 19;120(3):592-602. doi: 10.1182/blood-2012-02-408732. Epub 2012 Jun 1. Blood. 2012. PMID: 22661698 Free PMC article. - A role for the src family kinase Fyn in NK cell activation and the formation of the repertoire of Ly49 receptors.
Lowin-Kropf B, Kunz B, Schneider P, Held W. Lowin-Kropf B, et al. Eur J Immunol. 2002 Mar;32(3):773-82. doi: 10.1002/1521-4141(200203)32:3<773::AID-IMMU773>3.0.CO;2-U. Eur J Immunol. 2002. PMID: 11870621 - Regulation of NK cell responsiveness to achieve self-tolerance and maximal responses to diseased target cells.
Joncker NT, Raulet DH. Joncker NT, et al. Immunol Rev. 2008 Aug;224:85-97. doi: 10.1111/j.1600-065X.2008.00658.x. Immunol Rev. 2008. PMID: 18759922 Free PMC article. Review. - Selection, tuning, and adaptation in mouse NK cell education.
Kadri N, Thanh TL, Höglund P. Kadri N, et al. Immunol Rev. 2015 Sep;267(1):167-77. doi: 10.1111/imr.12330. Immunol Rev. 2015. PMID: 26284477 Review.
Cited by
- NK cells for cancer immunotherapy.
Shimasaki N, Jain A, Campana D. Shimasaki N, et al. Nat Rev Drug Discov. 2020 Mar;19(3):200-218. doi: 10.1038/s41573-019-0052-1. Epub 2020 Jan 6. Nat Rev Drug Discov. 2020. PMID: 31907401 Review. - Cytokine therapy restores antitumor responses of NK cells rendered anergic in MHC I-deficient tumors.
Ardolino M, Raulet DH. Ardolino M, et al. Oncoimmunology. 2015 Jun 5;5(1):e1002725. doi: 10.1080/2162402X.2014.1002725. eCollection 2016. Oncoimmunology. 2015. PMID: 26942049 Free PMC article. - The role and novel use of natural killer cells in graft-versus-leukemia reactions after allogeneic transplantation.
Hadjis AD, McCurdy SR. Hadjis AD, et al. Front Immunol. 2024 May 16;15:1358668. doi: 10.3389/fimmu.2024.1358668. eCollection 2024. Front Immunol. 2024. PMID: 38817602 Free PMC article. Review. - MHC Class I Deficiency in Solid Tumors and Therapeutic Strategies to Overcome It.
Shklovskaya E, Rizos H. Shklovskaya E, et al. Int J Mol Sci. 2021 Jun 23;22(13):6741. doi: 10.3390/ijms22136741. Int J Mol Sci. 2021. PMID: 34201655 Free PMC article. Review. - Activation receptor-induced tolerance of mature NK cells in vivo requires signaling through the receptor and is reversible.
Bolanos FD, Tripathy SK. Bolanos FD, et al. J Immunol. 2011 Mar 1;186(5):2765-71. doi: 10.4049/jimmunol.1003046. Epub 2011 Jan 24. J Immunol. 2011. PMID: 21263069 Free PMC article.
References
- Chalifour A., Scarpellino L., Back J., Brodin P., Devèvre E., Gros F., Lévy F., Leclercq G., Höglund P., Beermann F., Held W. 2009. A Role for cis Interaction between the Inhibitory Ly49A receptor and MHC class I for natural killer cell education. Immunity. 30:337–347 10.1016/j.immuni.2008.12.019 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials