Nuclear import of cytoplasmic poly(A) binding protein restricts gene expression via hyperadenylation and nuclear retention of mRNA - PubMed (original) (raw)
Nuclear import of cytoplasmic poly(A) binding protein restricts gene expression via hyperadenylation and nuclear retention of mRNA
G Renuka Kumar et al. Mol Cell Biol. 2010 Nov.
Abstract
Poly(A) tail length is emerging as an important marker of mRNA fate, where deviations from the canonical length can signal degradation or nuclear retention of transcripts. Pathways regulating polyadenylation thus have the potential to broadly influence gene expression. Here we demonstrate that accumulation of cytoplasmic poly(A) binding protein (PABPC) in the nucleus, which can occur during viral infection or other forms of cellular stress, causes mRNA hyperadenylation and nuclear accumulation of poly(A) RNA. This inhibits gene expression but does not affect mRNA stability. Unexpectedly, PABPC-induced hyperadenylation can occur independently of mRNA 3'-end processing yet requires the canonical mRNA poly(A) polymerase II. We find that nuclear PABPC-induced hyperadenylation is triggered by multiple divergent viral factors, suggesting that altering the subcellular localization of PABPC may be a commonly used mechanism to regulate cellular gene expression in a polyadenylation-linked manner.
Figures
FIG. 1.
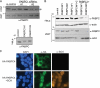
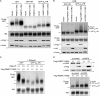
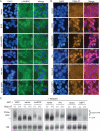
PABPC4 is induced upon PABPC1 depletion and is directed to the nucleus by SOX. (A) COS7, HEK 293T, and HeLa cells were transfected with either control siRNAs (si) or 2 independent siRNAs specific for PABPC1 (PABPC1#1 and PABPC1#2). siRNA transfections for COS7 and HEK 293T cells were performed in duplicate. At 72 h posttransfection, cell lysates were resolved by SDS-PAGE and immunoblotted with anti-PABPC antibodies. (B) HeLa and HEK 293T cells were transfected with control siRNAs or the indicated siRNAs specific for PABPC1 and/or PABPC4. Two independent PABPC4-specific siRNAs were used (PABPC4#1 and PABPC4#2). Lysates were then harvested and immunoblotted as described in the legend to panel A. (C) HEK 293T cells were transfected with a plasmid expressing HA-tagged PABPC4 alone or together with a plasmid expressing SOX and, 24 h later, subjected to immunofluorescence assays with anti-HA and anti-SOX antibodies. DAPI staining was used to visualize nuclei.
FIG. 2.
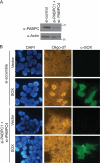
PABPC is required for SOX-induced nuclear poly(A) RNA accumulation. (A) HEK 293T cells were transfected with control siRNAs or siRNAs against PABPC1 and PABPC4. Lysates were harvested 24 h posttransfection and immunoblotted with anti-PABPC antibodies to detect the efficiency of PABPC protein depletion. In parallel, antiactin immunoblotting was performed to control for loading. (B) Cells described in the legend to panel A were transfected with either empty vector or a plasmid expressing SOX for 24 h and then processed for in situ hybridization with oligo(dT), followed by staining with anti-SOX antibodies and DAPI to visualize nuclei.
FIG. 3.
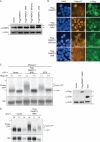
Nuclear accumulation of PABPC causes mRNA retention and hyperadenylation. (A) HEK 293T cells were transfected with the indicated PABPC1 expression plasmids for 24 h. Lysates were then harvested, resolved by SDS-PAGE, and immunoblotted with anti-PABPC antibodies to detect both endogenous (bottom arrow) and exogenous (upper arrow) PABPC proteins. Note that Flag-PABPC1 comigrates with endogenous PABPC1 in this blot. In parallel, antiactin immunoblotting was performed to control for loading. (B) HEK 293T cells were transfected as described in the legend to panel A for 24 h and then processed for oligo(dT) in situ hybridization, followed by immunofluorescence assays with anti-Flag antibodies. (C) HEK 293T cells were cotransfected with the indicated plasmids for 24 h. After treatment with leptomycin B (LMB) for 12 h to stabilize hyperadenylated species, total RNA and proteins were isolated. Total RNA was incubated in the presence or absence of oligo(dT) and digested with RNaseH. Products were resolved on a 1.2% agarose-formaldehyde gel and Northern blotted with 32P-labeled GFP and 18S probes (top). Hyperadenylated species are indicated by the labeled bracket [hyp(A) GFP]. Total protein was resolved by SDS-PAGE and immunoblotted using anti-Flag and anti-SOX antibodies (bottom). Actin served as a loading control. (D) Cells were transfected with the indicated plasmid as described above and treated with LMB for 7 h. Total RNA was then isolated from whole cells (W) or cytoplasmic fractions (C) and nuclear fractions (N) fractions and Northern blotted with 32P-labeled GFP and 18S probes. Hyperadenylated species are indicated by the labeled bracket [hyp(A) GFP].
FIG. 4.
Cytoplasmic PABPC and nuclear PABPC have opposing effects on gene expression. (A, B) HEK 293T cells were transfected with GFP and increasing amounts of Flag-PABPC1-CRS or Flag-PABPC1-NRS (100 to 900 ng) for 24 h. Total RNA was then isolated and visualized by Northern blotting with 32P-labeled GFP and 18S probes. Hyperadenylated species are indicated by the labeled bracket [hyp(A) GFP]. To monitor protein levels, protein lysate from the transfected cells was resolved by SDS-PAGE and immunoblotted with anti-Flag or antiactin (loading control) antibodies. (C) HEK 293T cells were transfected with GFP and empty vector, Flag-PABPC1-CRS, or Flag-PABPC1-NRS. At 24 h posttransfection, 5 μg/ml of actinomycin D was added to block transcription, and total RNA was harvested at the indicated time points thereafter. RNA was then visualized by Northern blotting with GFP and 18S probes, and the GFP mRNA half-life was calculated after 18S normalization. Error bars indicate standard errors between samples. Data were derived from five independent experiments. (D) HEK 293T cells were transfected with plasmids expressing GFP and empty vector, Flag-PABPC1-NRS, or Flag-PABPC1-CRS for 24 h. Equivalent amounts of protein lysate were resolved by SDS-PAGE and immunoblotted with antibodies against GFP, Flag, and actin (as a loading control).
FIG. 5.
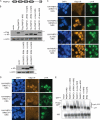
PABPC RRM1 and RRM2 are necessary and sufficient to induce hyperadenylation. (A) Diagram of PABPC, showing the 4 RRMs, followed by the linker region and conserved helical carboxyl terminus (square). (B) Expression levels from the indicated PABPC1 WT and mutant constructs were monitored following transfection into 293T cells for 24 h. Protein lysates harvested, resolved by SDS-PAGE, and immunoblotted with a mixture of anti-Flag and anti-HA antibodies or with antiactin antibody as a loading control. (C and D) HEK 293T cells were transfected with the indicated plasmids for 24 h and then subjected to oligo(dT) in situ hybridization and immunofluorescence assays with anti-HA antibodies. Nuclei were stained with DAPI. (E) HEK 293T cells were transfected with plasmids expressing GFP and either empty vector or the indicated PABPC construct for 24 h. Total RNA was then resolved by agarose-formaldehyde gel electrophoresis and Northern blotted with 32P-labeled GFP and 18S probes. Hyperadenylated species are indicated by the labeled bracket [hyp(A) GFP].
FIG. 6.
Cellular mRNA 3′-end processing enhances but is not required for PABPC-induced hyperadenylation. (A) HEK 293T cells were transfected with the indicated plasmids for 24 h, and then total RNA was isolated and Northern blotted with 32P-labeled GFP and 18S probes. (B) Same protocol as described in the legend to panel A, but cells were treated with 5 ng/ml LMB for 12 h prior to RNA isolation to enhance detection of hyperadenylated species. (C) HEK 293T cells were transfected with the indicated plasmids for 24 h and then treated with 5 ng/ml LMB for 12 h. Total RNA was isolated and incubated in the presence or absence of oligo(dT) and then digested with RNaseH. RNA was visualized by Northern blotting with 32P-labeled GFP and 18S probes. (D) HEK 293T cells were transfected twice over 48 h with either PAPII siRNAs or control siRNAs and then subsequently transfected in duplicate with DNA plasmids expressing GFP-A60-HR alone or together with Flag-PABPC1-NRS for 24 h. Samples were treated with 5 ng/ml LMB for 6 h prior to harvesting either protein (top) or RNA (bottom). Protein lysates were resolved by SDS-PAGE and immunoblotted with antibodies against PAPII, Flag, or actin (as a loading control). RNA from each sample was Northern blotted with 32P-labeled GFP and 18S probes.
FIG. 7.
Nuclear relocalization of PABPC, nuclear retention of mRNA, and hyperadenylation are phenotypes induced by multiple independent viral factors. (A) HEK 293T cells were transfected with either empty vector or with plasmids expressing SOX, HA-muSOX, vhs, vhs mutant (vhs-mut), or SARS-CoV NSP1 for 24 h and then subjected to immunofluorescence assays (IFA) with anti-PABPC antibodies and stained with DAPI to visualize nuclei. Right panels represent a merge between the IFA and DAPI signals. (B) HEK 293T cells were transfected as described in the legend to panel A, subjected to oligo(dT) in situ hybridization, and stained with DAPI. Right panels represent a merge between the in situ and DAPI signals. (C) HEK 293T cells were cotransfected with a plasmid expressing GFP and either empty vector or plasmids expressing the indicated viral protein for 24 h. Cells were treated with LMB for 15 h, and total RNA was then isolated and digested with RNaseH in the presence or absence of oligo(dT). Products were resolved by agarose-formaldehyde gel electrophoresis and Northern blotted with 32P-labeled GFP and 18S probes.
Similar articles
- Importin alpha-mediated nuclear import of cytoplasmic poly(A) binding protein occurs as a direct consequence of cytoplasmic mRNA depletion.
Kumar GR, Shum L, Glaunsinger BA. Kumar GR, et al. Mol Cell Biol. 2011 Aug;31(15):3113-25. doi: 10.1128/MCB.05402-11. Epub 2011 Jun 6. Mol Cell Biol. 2011. PMID: 21646427 Free PMC article. - The nuclear poly(A) binding protein of mammals, but not of fission yeast, participates in mRNA polyadenylation.
Kühn U, Buschmann J, Wahle E. Kühn U, et al. RNA. 2017 Apr;23(4):473-482. doi: 10.1261/rna.057026.116. Epub 2017 Jan 17. RNA. 2017. PMID: 28096519 Free PMC article. - A conserved CCCH-type zinc finger protein regulates mRNA nuclear adenylation and export.
Hurt JA, Obar RA, Zhai B, Farny NG, Gygi SP, Silver PA. Hurt JA, et al. J Cell Biol. 2009 Apr 20;185(2):265-77. doi: 10.1083/jcb.200811072. Epub 2009 Apr 13. J Cell Biol. 2009. PMID: 19364924 Free PMC article. - Poly(A) RNA-binding proteins and polyadenosine RNA: new members and novel functions.
Wigington CP, Williams KR, Meers MP, Bassell GJ, Corbett AH. Wigington CP, et al. Wiley Interdiscip Rev RNA. 2014 Sep-Oct;5(5):601-22. doi: 10.1002/wrna.1233. Epub 2014 Apr 30. Wiley Interdiscip Rev RNA. 2014. PMID: 24789627 Free PMC article. Review. - Virus-mediated mRNA decay by hyperadenylation.
Sokoloski KJ, Chaskey EL, Wilusz J. Sokoloski KJ, et al. Genome Biol. 2009;10(8):234. doi: 10.1186/gb-2009-10-8-234. Epub 2009 Aug 11. Genome Biol. 2009. PMID: 19678916 Free PMC article. Review.
Cited by
- RNase L limits host and viral protein synthesis via inhibition of mRNA export.
Burke JM, Gilchrist AR, Sawyer SL, Parker R. Burke JM, et al. Sci Adv. 2021 Jun 4;7(23):eabh2479. doi: 10.1126/sciadv.abh2479. Print 2021 Jun. Sci Adv. 2021. PMID: 34088676 Free PMC article. - A viral nuclear noncoding RNA binds re-localized poly(A) binding protein and is required for late KSHV gene expression.
Borah S, Darricarrère N, Darnell A, Myoung J, Steitz JA. Borah S, et al. PLoS Pathog. 2011 Oct;7(10):e1002300. doi: 10.1371/journal.ppat.1002300. Epub 2011 Oct 13. PLoS Pathog. 2011. PMID: 22022268 Free PMC article. - Mechanisms and consequences of mRNA destabilization during viral infections.
Shehata SI, Watkins JM, Burke JM, Parker R. Shehata SI, et al. Virol J. 2024 Feb 6;21(1):38. doi: 10.1186/s12985-024-02305-1. Virol J. 2024. PMID: 38321453 Free PMC article. Review. - Birth of a poly(A) tail: mechanisms and control of mRNA polyadenylation.
Rodríguez-Molina JB, Turtola M. Rodríguez-Molina JB, et al. FEBS Open Bio. 2023 Jul;13(7):1140-1153. doi: 10.1002/2211-5463.13528. Epub 2022 Dec 7. FEBS Open Bio. 2023. PMID: 36416579 Free PMC article. Review. - Coordinated destruction of cellular messages in translation complexes by the gammaherpesvirus host shutoff factor and the mammalian exonuclease Xrn1.
Covarrubias S, Gaglia MM, Kumar GR, Wong W, Jackson AO, Glaunsinger BA. Covarrubias S, et al. PLoS Pathog. 2011 Oct;7(10):e1002339. doi: 10.1371/journal.ppat.1002339. Epub 2011 Oct 27. PLoS Pathog. 2011. PMID: 22046136 Free PMC article.
References
- Afonina, E., R. Stauber, and G. N. Pavlakis. 1998. The human poly(A)-binding protein 1 shuttles between the nucleus and the cytoplasm. J. Biol. Chem. 273:13015-13021. - PubMed
- Anderson, J. T., and X. Wang. 2009. Nuclear RNA surveillance: no sign of substrates tailing off. Crit. Rev. Biochem. Mol. Biol. 44:16-24. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources