Functional control of transplantable human ESC-derived neurons via optogenetic targeting - PubMed (original) (raw)
Functional control of transplantable human ESC-derived neurons via optogenetic targeting
Jason P Weick et al. Stem Cells. 2010 Nov.
Abstract
Current methods to examine and regulate the functional integration and plasticity of human ESC (hESC)-derived neurons are cumbersome and technically challenging. Here, we engineered hESCs and their derivatives to express the light-gated channelrhodopsin-2 (ChR2) protein to overcome these deficiencies. Optogenetic targeting of hESC-derived neurons with ChR2 linked to the mCherry fluorophore allowed reliable cell tracking as well as light-induced spiking at physiological frequencies. Optically induced excitatory and inhibitory postsynaptic currents could be elicited in either ChR2(+) or ChR2(-) neurons in vitro and in acute brain slices taken from transplanted severe combined immunodeficient (SCID) mice. Furthermore, we created a clonal hESC line that expresses ChR2-mCherry under the control of the synapsin-1 promoter. On neuronal differentiation, ChR2-mCherry expression was restricted to neurons and was stably expressed for at least 6 months, providing more predictable light-induced currents than transient infections. This pluripotent cell line will allow both in vitro and in vivo analysis of functional development as well as the integration capacity of neuronal populations for cell-replacement strategies.
Copyright © 2010 AlphaMed Press.
Figures
Figure 1
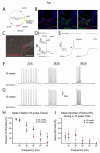
Fidelity of ChR2-induced spiking depends on developmental age in hESC-derived neurons. (A) Diagram of lentiviral targeting vector with ChR2 driven by the synapsin-1 promoter and linked to the mCherry fluorophore. (B) Confocal z-stacks of cells expressing ChR2-mCherry colocalized with synapsin-1 protein, but not in synapsin-1− cells. (C) Merged DIC and fluorescent image of a recorded ChR2-mCherry+ cell. (D) Representative traces from voltage-clamp (i) and current-clamp (ii) recordings of ChR2+ cell during a 500ms, 470nm light stimulus. (E) Expanded timescale of current-clamp recording which demonstrates the presence of an action potential (AP) by a clear threshold deflection (arrow) and the ability to inhibit the AP by 1μM Tetrodotoxin (right trace). (F-G) Representative current-clamp traces recorded during 470nm light stimulations of 5, 10, and 20Hz for cells differentiated for 10 weeks (F) and 25 weeks (G). (H) Pooled data demonstrate significant increases in the fidelity of optically-induced AP generation in 25 week-old neurons at frequencies of 20-50Hz. See also Supplemental Figure 1. (I) Chart depicting the mean number of extra spikes fired during 10-pulse trains. *p<0.05. Error bars indicate SEM. Scale bars represent 50μm.
Figure 2
ChR2-induced post-synaptic currents (PSCs) in hESC-derived neurons in vitro. (A) Voltage-clamp recording of a ChR2+ cell illustrates the presence of PSCs following escape action current (AC) generation (upper left trace) and in response to ChR2 stimulation alone (upper right trace). See also Supplemental Figure 2A. PSCs recorded after an escape AC or in the absence of an AC could be blocked by a combination of AMPA and GABA receptor antagonists CNQX and bicuculine (BIC), respectively (lower traces). (B) Combined DIC/fluorescent image of a ChR2− cell recorded adjacent to ChR2+ cells. (C) Excitatory (i, upper trace) and inhibitory (ii, upper trace) PSCs could be generated in two different ChR2− cells, which were blocked by CNQX (i, lower trace) or bicuculine (ii, lower trace), respectively. (D) Expanded timescale of an individual PSC to illustrate the temporal delay between light stimulus (blue bar) and PSC onset. See also Supplemental Figure 2B. Scale bar represents 50μm.
Figure 3
ChR2-induced currents in hESC-derived neurons in acute slice preparations. (A) DIC/fluorescent image of a hESC-derived neuron recorded in an acute slice preparation from a 5 month-old SCID mouse. (B) Current-clamp traces demonstrating the presence of spontaneous AP generation. (C) Voltage-clamp recordings demonstrating the presence of spontaneous PSCs with different temporal kinetics, indicative of both excitatory and inhibitory synapses. (D) Representative current-clamp traces of ChR2-induced spiking at 5Hz (upper) and 10Hz (lower) frequencies in response to 470nm light stimulation. (E) 5Hz train of APs generated in response to light stimulus and recorded in the on-cell configuration, demonstrating the ability of hESC-derived neurons to generate mature spikes (inset) without experimental alteration of resting membrane potential. (F) Representative traces from ChR2− cells that showed PSCs in response to light stimulation of the slice. Inset illustrates the temporal delay between light stimulus (blue bar) and PSC onset. Scale bar represents 20μm.
Figure 4
A clonal Synapsin-ChR2-mCherry cell line expresses mCherry upon neuronal differentiation. Paired Hoffman contrast and epifluorescent images of differentiating Syn-ChR2-mCherry cells illustrate a lack of ChR2-mCherry expression as embryonic stem cells (ES cells, day 0) and during the embryoid body stage (EBs, days 1-7). Cells remain largely devoid of expression as floating neuroepithelia (NE, days 7-21), but displayed robust mCherry expression in post-mitotic neurons (PMNs) at 25 days (column 4 panels) and 80 days in vitro (column 5 panels). Scale bars indicate 100μm.
Figure 5
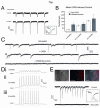
Synapsin-ChR2-mCherry cell line exhibits a full complement of optically-induced currents. (A) Representative traces of ChR2 currents from Syn-ChR2 neurons at 5 and 10 weeks; inset shows light-current relationship. (B) Pooled data demonstrates that the ChR2-mediated currents in the cell line increased during differentiation in response to 10ms light stimuli, and displayed less variation compared with hESC-derived neurons that were infected upon differentiation. (C) Representative voltage-clamp traces from Syn-ChR2-derived neurons after 10 weeks of differentiation illustrate the presence of ePSCs and iPSCs. ePSCs were specifically blocked by application of CNQX (50μM) and iPSCs were blocked by bicuculine (20μM). (D) Representative current-clamp traces from Syn-ChR2-mCherry neurons in response to 10Hz stimulations at 6 weeks (i), 12 weeks (ii) and 4 months (iii) of differentiation. (E) Paired Hoffman contrast and fluorescent images of co-cultures of wild-type hESC-derived neurons and Syn-ChR2-mCherry neurons. Representative voltage-clamp recording of a ChR2− cell that generated post-synaptic currents in response to 470nm light stimuli (upper trace), and could be blocked with bicuculine (lower trace). Inset shows light-current relationship. Error bars represent SEM. Scale bars indicate 100μm.
Similar articles
- Optogenetic control of iPS cell-derived neurons in 2D and 3D culture systems using channelrhodopsin-2 expression driven by the synapsin-1 and calcium-calmodulin kinase II promoters.
Lee SY, George JH, Nagel DA, Ye H, Kueberuwa G, Seymour LW. Lee SY, et al. J Tissue Eng Regen Med. 2019 Mar;13(3):369-384. doi: 10.1002/term.2786. Epub 2019 Jan 30. J Tissue Eng Regen Med. 2019. PMID: 30550638 Free PMC article. - Tracking stem cell differentiation in the setting of automated optogenetic stimulation.
Stroh A, Tsai HC, Wang LP, Zhang F, Kressel J, Aravanis A, Santhanam N, Deisseroth K, Konnerth A, Schneider MB. Stroh A, et al. Stem Cells. 2011 Jan;29(1):78-88. doi: 10.1002/stem.558. Stem Cells. 2011. PMID: 21280159 Free PMC article. - Optogenetically transduced human ES cell-derived neural progenitors and their neuronal progenies: Phenotypic characterization and responses to optical stimulation.
Ryu J, Vincent PFY, Ziogas NK, Xu L, Sadeghpour S, Curtin J, Alexandris AS, Stewart N, Sima R, du Lac S, Glowatzki E, Koliatsos VE. Ryu J, et al. PLoS One. 2019 Nov 11;14(11):e0224846. doi: 10.1371/journal.pone.0224846. eCollection 2019. PLoS One. 2019. PMID: 31710637 Free PMC article. - Human embryonic stem cell-derived neurons adopt and regulate the activity of an established neural network.
Weick JP, Liu Y, Zhang SC. Weick JP, et al. Proc Natl Acad Sci U S A. 2011 Dec 13;108(50):20189-94. doi: 10.1073/pnas.1108487108. Epub 2011 Nov 21. Proc Natl Acad Sci U S A. 2011. PMID: 22106298 Free PMC article. - Channelrhodopsin as a tool to investigate synaptic transmission and plasticity.
Schoenenberger P, Schärer YP, Oertner TG. Schoenenberger P, et al. Exp Physiol. 2011 Jan;96(1):34-9. doi: 10.1113/expphysiol.2009.051219. Epub 2010 Jun 18. Exp Physiol. 2011. PMID: 20562296 Review.
Cited by
- Channelrhodopsins: visual regeneration and neural activation by a light switch.
G N, Tan A, Farhatnia Y, Rajadas J, Hamblin MR, Khaw PT, Seifalian AM. G N, et al. N Biotechnol. 2013 Jun 25;30(5):461-74. doi: 10.1016/j.nbt.2013.04.007. Epub 2013 May 7. N Biotechnol. 2013. PMID: 23664865 Free PMC article. Review. - Probing Neural Transplant Networks In Vivo with Optogenetics and Optogenetic fMRI.
Weitz AJ, Lee JH. Weitz AJ, et al. Stem Cells Int. 2016;2016:8612751. doi: 10.1155/2016/8612751. Epub 2016 May 12. Stem Cells Int. 2016. PMID: 27293449 Free PMC article. Review. - Modeling complex neuropsychiatric disease with induced pluripotent stem cells.
Ross PJ, Ellis J. Ross PJ, et al. F1000 Biol Rep. 2010 Dec 8;2:84. doi: 10.3410/B2-84. F1000 Biol Rep. 2010. PMID: 21283649 Free PMC article. - Effect of optogenetic stimulus on the proliferation and cell cycle progression of neural stem cells.
Wang SJ, Weng CH, Xu HW, Zhao CJ, Yin ZQ. Wang SJ, et al. J Membr Biol. 2014 Jun;247(6):493-500. doi: 10.1007/s00232-014-9659-7. Epub 2014 Apr 19. J Membr Biol. 2014. PMID: 24748510 - Optogenetic stimulation inhibits the self-renewal of mouse embryonic stem cells.
Wang S, Du L, Peng GH. Wang S, et al. Cell Biosci. 2019 Sep 3;9:73. doi: 10.1186/s13578-019-0335-6. eCollection 2019. Cell Biosci. 2019. PMID: 31497278 Free PMC article.
References
- Ladewig J, Koch P, Endl E, et al. Lineage selection of functional and cryopreservable human embryonic stem cell-derived neurons. STEM CELLS. 2008;26:1705–1712. - PubMed
- Sonntag KC, Simantov R, Isacson O. Stem cells may reshape the prospect of Parkinson's disease therapy. BRAIN RES MOL BRAIN RES. 2005;134:34–51. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P30 HD003352/HD/NICHD NIH HHS/United States
- NS045926/NS/NINDS NIH HHS/United States
- T32 GM008692/GM/NIGMS NIH HHS/United States
- R01 NS045926/NS/NINDS NIH HHS/United States
- R21 NS061243/NS/NINDS NIH HHS/United States
- P01 NS057778/NS/NINDS NIH HHS/United States
- P30 HD03352/HD/NICHD NIH HHS/United States
- NS057778/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases