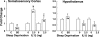ATP and the purine type 2 X7 receptor affect sleep - PubMed (original) (raw)
ATP and the purine type 2 X7 receptor affect sleep
James M Krueger et al. J Appl Physiol (1985). 2010 Nov.
Abstract
Sleep is dependent upon prior brain activities, e.g., after prolonged wakefulness sleep rebound occurs. These effects are mediated, in part, by humoral sleep regulatory substances such as cytokines. However, the property of wakefulness activity that initiates production and release of such substances and thereby provides a signal for indexing prior waking activity is unknown. We propose that extracellular ATP, released during neuro- and gliotransmission and acting via purine type 2 (P2) receptors, is such a signal. ATP induces cytokine release from glia. Cytokines in turn affect sleep. We show here that a P2 receptor agonist, 2'(3')-O-(4-benzoylbenzoyl)adenosine 5'-triphosphate (BzATP), increased non-rapid eye movement sleep (NREMS) and electroencephalographic (EEG) delta power while two different P2 receptor antagonists, acting by different inhibitory mechanisms, reduced spontaneous NREMS in rats. Rat P2X7 receptor protein varied in the somatosensory cortex with time of day, and P2X7 mRNA was altered by interleukin-1 treatment, by sleep deprivation, and with time of day in the hypothalamus and somatosensory cortex. Mice lacking functional P2X7 receptors had attenuated NREMS and EEG delta power responses to sleep deprivation but not to interleukin-1 treatment compared with wild-type mice. Data are consistent with the hypothesis that extracellular ATP, released as a consequence of cell activity and acting via P2 receptors to release cytokines and other sleep regulatory substances, provides a mechanism by which the brain could monitor prior activity and translate it into sleep.
Figures
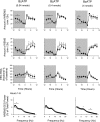
Fig. 1.
Nighttime (shaded area) injection of 2′(3′)-_O_-(4-benzoylbenzoyl)adenosine 5′-triphosphate (BzATP) enhanced non-rapid eye movement sleep (NREMS). Rats received 1 of 3 doses of BzATP as indicated at dark onset. All 3 doses enhanced duration of NREMS during the initial 6–8 h after injection. The highest dose also enhanced duration of NREMS in the subsequent light period. BzATP inhibited duration of rapid eye movement sleep (REMS) during the daylight period occurring 12 h after the injection after the lower 2 doses. After the highest dose, REMS was enhanced during the first 4 h after injection. BzATP induced enhancement of electroencephalographic slow-wave activity (EEG SWA). BzATP at the lowest dose enhanced EEG delta power; this effect was absent after the higher doses. EEG power values were obtained during the first 6 h after injection. ○, Control values; ●, values obtained after BzATP injections. *Significant differences between control and test days (P < 0.05, paired _t_-test).
Fig. 2.
Daytime (light areas) injection of BzATP enhanced duration of rat NREMS, EEG SWA during NREMS, and EEG spectral power. In contrast, duration of REMS was decreased during daylight hours but enhanced in the subsequent dark period. EEG power values were obtained during postinjection hours 1–6. ○, Values from control recordings; ●, values obtained after BzATP injections. *Significant differences between control and test days (P < 0.05, paired _t_-test).
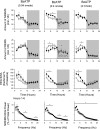
Fig. 3.
Both P2X7 receptor antagonists, injected at light onset (filled bars, ●), suppressed NREMS compared with saline injections (open bars, ○). oxidized ATP (OxATP) attenuated SWA during the first 12 h at the 1–4 Hz frequencies. Although EEG SWA was not significantly affected by A438079 during the first 6 h. With select 2-h time bins A438079 induced a biphasic effect, initially suppressing but then augmenting the EEG power spectra at different frequencies (see text for details, data not shown). Two smaller doses of A438079 were also tested, 1 nmol and 10 nmol; neither dose affected any of the parameters measured (*P < 0.05 between control and test days).
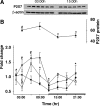
Fig. 4.
Diurnal variation in the relative amount of interleukin-1β (IL1) mRNA and P2X7 receptor mRNA and protein in the hypothalamus and somatosensory cortex. A: Western blot analyses of somatosensory cortex samples showed variation of P2X7 receptor protein levels at 1500 (3 h after light onset) or 0300 (3 h after dark onset). No differences in time of day were observed in the standard, β-actin. B: time course of P2X7 receptor mRNA and protein (▴) and IL1 mRNA (●). The levels of P2X7 receptor protein (somatosensory cortex) and mRNA [(hypothalamus (□) and somatosensory cortex (▼)] were greater during the light period than during the dark period (black horizontal bar indicates dark period). Expression of protein levels was normalized to β-actin levels. *Statistical significance (P < 0.05) from values obtained at 2100; +significant differences from dark onset; #significant differences from values obtained 12 h before.
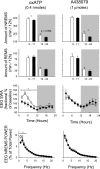
Fig. 5.
Expression of P2X7 receptor mRNA in the somatosensory cortex and hypothalamus after 6 h of sleep deprivation (SD) or 2 h and 5 h after intracerebroventricular injection of 2.5 ng of IL1. Left: SD significantly decreased the levels of P2X7 receptor mRNA in the somatosensory cortex, and IL1 enhanced P2X7 mRNA levels both 2 and 5 h after the injection. Right: in the hypothalamus SD enhanced P2X7 mRNA levels, and IL1 significantly decreased P2X7 receptor mRNA. *Significant difference from corresponding control (C).
Fig. 6.
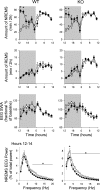
Sleep responses induced by sleep deprivation were attenuated in P2X7 receptor knockout (KO) mice compared with wild type (WT) control mice. Mice were deprived of sleep from 0600 to 1200 and then allowed to sleep ad libitum. WT mice exhibited typical NREMS rebound (right, inset) and EEG SWA enhancements (left) during the first 2 h after deprivation as previously reported (24). In contrast, P2X7 receptor KO mice had attenuated NREMS and EEG SWA responses during the same period. Both strains had reduced EEG SWA for postdeprivation hours 6–22 (1800–1000) as previously reported (24). *P < 0.05 difference between WT and KO mice for NREMS and EEG SWA. ○, Baseline values; ●, values after sleep deprivation.
Fig. 7.
IL1 enhances duration of NREMS and inhibits EEG SWA during NREMS after intraperitoneal injections in P2X7 receptor KO and WT mice. ●, Data from IL1-treated mice; ○, values from saline-treated control mice. Unlike intracerebroventricular or intravenous injections in rats, intraperitoneal injections of IL1 reduced EEG power as previously reported (37). The responses to IL1 were similar in both genotypes (WT, left; KO, right), suggesting that IL1's influence on sleep results from a step downstream from the ATP-P2 receptor interaction. *P < 0.05 difference between baseline and test days.
Fig. 8.
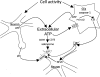
Extracellular ATP involvement in sleep regulation. ATP is released into the extracellular space as a consequence of cell activity during neuro- or gliotransmission. Extracellular ATP then activates P2 receptors (R), e.g., P2X7, that in turn are involved in IL1 processing and release as well as release of other sleep regulatory substances such as tumor necrosis factor and brain-derived neurotrophic factor (not shown). IL1, in turn, activates nuclear factor-κB (NF-κB), leading to changes in receptor trafficking. This changes the cell's long-term sensitivity to neurotransmitters such as glutamate (glu) and to neuromodulators such as adenosine. Extracellular ATP is also catabolized to adenosine via the actions of ectonucleotidases such as CD39 and CD73; this action is faster than the ATP-P2-induced changes in transcription and translation. Both actions of ATP are likely involved in sleep regulation. AMPA, amino-3-hydroxy-5-methyl-4-isoxazole proprionic acid.
Similar articles
- Ecto-nucleotidases and nucleoside transporters mediate activation of adenosine receptors on hippocampal mossy fibers by P2X7 receptor agonist 2'-3'-O-(4-benzoylbenzoyl)-ATP.
Kukley M, Stausberg P, Adelmann G, Chessell IP, Dietrich D. Kukley M, et al. J Neurosci. 2004 Aug 11;24(32):7128-39. doi: 10.1523/JNEUROSCI.2093-04.2004. J Neurosci. 2004. PMID: 15306646 Free PMC article. - Involvement of cytokines in slow wave sleep.
Krueger JM, Clinton JM, Winters BD, Zielinski MR, Taishi P, Jewett KA, Davis CJ. Krueger JM, et al. Prog Brain Res. 2011;193:39-47. doi: 10.1016/B978-0-444-53839-0.00003-X. Prog Brain Res. 2011. PMID: 21854954 Free PMC article. Review. - Activation of basal forebrain purinergic P2 receptors promotes wakefulness in mice.
Yang C, Larin A, McKenna JT, Jacobson KA, Winston S, Strecker RE, Kalinchuk A, Basheer R, Brown RE. Yang C, et al. Sci Rep. 2018 Jul 16;8(1):10730. doi: 10.1038/s41598-018-29103-4. Sci Rep. 2018. PMID: 30013200 Free PMC article. - P2X7-related modulation of pathological nociception in rats.
McGaraughty S, Chu KL, Namovic MT, Donnelly-Roberts DL, Harris RR, Zhang XF, Shieh CC, Wismer CT, Zhu CZ, Gauvin DM, Fabiyi AC, Honore P, Gregg RJ, Kort ME, Nelson DW, Carroll WA, Marsh K, Faltynek CR, Jarvis MF. McGaraughty S, et al. Neuroscience. 2007 Jun 8;146(4):1817-28. doi: 10.1016/j.neuroscience.2007.03.035. Epub 2007 May 3. Neuroscience. 2007. PMID: 17478048 - Delta wave power: an independent sleep phenotype or epiphenomenon?
Davis CJ, Clinton JM, Jewett KA, Zielinski MR, Krueger JM. Davis CJ, et al. J Clin Sleep Med. 2011 Oct 15;7(5 Suppl):S16-8. doi: 10.5664/JCSM.1346. J Clin Sleep Med. 2011. PMID: 22003323 Free PMC article. Review.
Cited by
- Sleep and Microbes.
Krueger JM, Opp MR. Krueger JM, et al. Int Rev Neurobiol. 2016;131:207-225. doi: 10.1016/bs.irn.2016.07.003. Epub 2016 Aug 31. Int Rev Neurobiol. 2016. PMID: 27793219 Free PMC article. Review. - Circadian rhythm and sleep-wake systems share the dynamic extracellular synaptic milieu.
Cooper JM, Halter KA, Prosser RA. Cooper JM, et al. Neurobiol Sleep Circadian Rhythms. 2018 Apr 17;5:15-36. doi: 10.1016/j.nbscr.2018.04.001. eCollection 2018 Jun. Neurobiol Sleep Circadian Rhythms. 2018. PMID: 31236509 Free PMC article. Review. - From Physiology to Pathology of Cortico-Thalamo-Cortical Oscillations: Astroglia as a Target for Further Research.
Gobbo D, Scheller A, Kirchhoff F. Gobbo D, et al. Front Neurol. 2021 Jun 9;12:661408. doi: 10.3389/fneur.2021.661408. eCollection 2021. Front Neurol. 2021. PMID: 34177766 Free PMC article. Review. - Leukocyte Expression of Type 1 and Type 2 Purinergic Receptors and Pro-Inflammatory Cytokines during Total Sleep Deprivation and/or Sleep Extension in Healthy Subjects.
Chennaoui M, Arnal PJ, Drogou C, Leger D, Sauvet F, Gomez-Merino D. Chennaoui M, et al. Front Neurosci. 2017 May 2;11:240. doi: 10.3389/fnins.2017.00240. eCollection 2017. Front Neurosci. 2017. PMID: 28512397 Free PMC article. - Augmented generation of protein fragments during wakefulness as the molecular cause of sleep: a hypothesis.
Varshavsky A. Varshavsky A. Protein Sci. 2012 Nov;21(11):1634-61. doi: 10.1002/pro.2148. Protein Sci. 2012. PMID: 22930402 Free PMC article.
References
- Appelbaum L, Skariah G, Mourrain P, Mignot E. Comparative expression of p2x receptors and ecto-nucleoside triphosphate diphosphohydrolase 3 in hypocretin and sensory neurons in zebrafish. Brain Res 1174: 66–75, 2007 - PubMed
- Belcher SM, Zsarnovszky A, Crawford PA, Hemani H, Spurling L, Kirley TL. Immunolocalization of ecto-nucleoside triphosphate diphosphohydrolase 3 in rat brain: implications for modulation of multiple homeostatic systems including feeding and sleep-wake behaviors. Neuroscience 137: 1331–1346, 2006 - PubMed
- Bianco F, Pravettoni E, Colombi A, Schenk U, Moller T, Matteoli M, Verderia C. Astrocyte-derived ATP induces vesicle shedding and IL-1beta release from microglia. J Immunol 174: 7268–7677, 2005 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources