Functional coupling between the extracellular matrix and nuclear lamina by Wnt signaling in progeria - PubMed (original) (raw)
. 2010 Sep 14;19(3):413-25.
doi: 10.1016/j.devcel.2010.08.013.
Kyle J Roux, Esther Sook Miin Wong, Leslie C Mounkes, Rafidah Mutalif, Raju Navasankari, Bina Rai, Simon Cool, Jae-Wook Jeong, Honghe Wang, Hyun-Shik Lee, Serguei Kozlov, Martin Grunert, Thomas Keeble, C Michael Jones, Margarita D Meta, Stephen G Young, Ira O Daar, Brian Burke, Alan O Perantoni, Colin L Stewart
Affiliations
- PMID: 20833363
- PMCID: PMC2953243
- DOI: 10.1016/j.devcel.2010.08.013
Functional coupling between the extracellular matrix and nuclear lamina by Wnt signaling in progeria
Lidia Hernandez et al. Dev Cell. 2010.
Abstract
The segmental premature aging disease Hutchinson-Gilford Progeria (HGPS) is caused by a truncated and farnesylated form of Lamin A. In a mouse model for HGPS, a similar Lamin A variant causes the proliferative arrest and death of postnatal, but not embryonic, fibroblasts. Arrest is due to an inability to produce a functional extracellular matrix (ECM), because growth on normal ECM rescues proliferation. The defects are associated with inhibition of canonical Wnt signaling, due to reduced nuclear localization and transcriptional activity of Lef1, but not Tcf4, in both mouse and human progeric cells. Defective Wnt signaling, affecting ECM synthesis, may be critical to the etiology of HGPS because mice exhibit skeletal defects and apoptosis in major blood vessels proximal to the heart. These results establish a functional link between the nuclear envelope/lamina and the cell surface/ECM and may provide insights into the role of Wnt signaling and the ECM in aging.
Copyright © 2010 Elsevier Inc. All rights reserved.
Figures
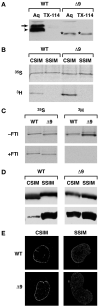
Figure 1. Δ9LaminA is farnesylated and not processed to a mature form
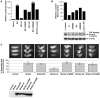
A: WT LMNA tail partitions to the aqueous phase (Aq), not the detergent phase (TX-114). PrelaminA (arrow) is distinct from mature LMNA (arrowhead). The LMNAΔ9 tail is a single band (*) and partitions to both aqueous and detergent phases. B-C: HA-tagged full-length CSIM and SSIM variants of LMNA WT and Δ9 were translated with 35S-labeled cysteine and methionine or 3H-labeled-mevalonolactone in the presence (C) or absence of 20μM farnesyltransferase inhibitor PB-43. D: HA- or myc-tagged CSIM or SSIM variants of full-length human LMNA-WT and Δ9 were immunoblotted with anti-HA or Myc (top panel) or prelamin A antibodies (lower panels). LMNAΔ9-CSIM is similar in size to the SSIM variant (top right panel). CSIM WT-LMNA is processed and migrates faster than the SSIM variant (top left panel). E: NE vs. nucleoplasmic localization of CSIM and SSIM variants of LMNA-WT and Δ9 was analyzed by immunofluorescence in _Lmna_−/− MEFs.
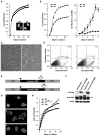
Figure 2. Δ9 and Δ50 result in abnormal nuclear morphologies and accelerated proliferative arrest
A: WT (■) and Δ9 (●) MEF growth curves, with nuclear morphology shown in the insets (DAPI staining). B: WT (■) and Δ9 (●) MAF growth curves. C: Passage 4-5 WT (left panels) and Δ9 (right panels) MAFs assayed for morphology (C) and apoptosis (D). E: Retroviral vectors for Δ9 or Δ50 HA-tagged LMNA cDNAs. HA tag (black triangle) and deletions (open triangles). F: Western analysis of retroviral protein production. WT-LMNA is not HA tagged; all Lamins are detected by the Jol2 monoclonal antibody specific for hLMNA. The Δ50hLMNA band (arrow) migrates just above a non-specific band detected in all samples by Jol2. G: Jol-2 immunostaining reveals nuclear morphology in cells expressing Progerin/Δ50 (G1), Δ9 (G2) and Wt Lmna(G3). H; _Lmna_−/− cells proliferate and immortalize. Growth curves show the effect of reintroduction of LMNA constructs.
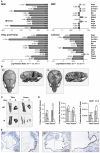
Figure 3. Δ9 mice show reduced ECM gene expression and abnormal skeletal and vascular development
A: Confirmation of the microarray analysis (Tables S1, 2a-c and Figures S2a-b) by RQ-PCR. Differences are expressed as the Relative Log Ratio between the Δ9 and Wt samples, with a 2-fold decrease being equivalent to −0.301, 4-fold decrease −0.602, 5-fold decrease −0.699, p*<0.05, **<0.01 and ***<0.005 at the 95% confidence interval. Error bars are S.E.M. B-D: Calvarial (B) and long bone (C) mineral density, as well as long bone volume and trabecular thickness and number (D) in P14 WT and Δ9 sibling mice; p*<0.05, **<0.01 and ***<0.005 (3 of each genotype) E: Apoptosis in pulmonary artery smooth muscle of P16 WT and Δ9 mice (TUNEL-staining indicated by arrows).
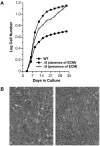
Figure 4. Δ9MAF growth is rescued by WT extracellular matrix
A: Growth curves of WT MAFs and of Δ9MAFs in the presence or absence of WT MAF ECM. B: Left panel Δ9MAFS at p4 with no ECM, Right panel Δ9MAFs on ECM. Growth of Δ9MAFs on specific ECM components or with FTIs is shown in Figures S3a-b.
Figure 5. Δ9 and Δ50 Lamin inhibit Wnt mediated transcription
A: TOPflash/FOPflash luciferase reporter assays with WT (BC), Δ9, Δ50 lamins or their SSIM variants. B: Gst-Tcf pull down of nuclear β-catenin levels. C. Injection of Xwnt3a mRNA into Xenopus embryos, alone or in the presence of each LMNA construct shown in panels A and B. Lower panel Western shows amount of protein derived from the different LMNA mRNAs injected.
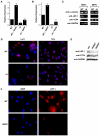
Figure 6. Lef1 levels are reduced in Δ9 and Progeric cells
A: ChIP analysis of Lef1 binding to the Col11A1 promoter in WT and Δ9 MAFs, in the presence an absence of pre-treatment with the SB415286 Gsk-3β inhibitor (10μm). B: RT-PCR detection of Lef1 mRNA in WT and Δ_9_MAFs. C: Western analysis of β-catenin, Lef1, and Tcf4 in WT and Δ9MAFs and MEFs. D: Immunofluorescent detection of Lef1 and Tcf4 in WT and Δ9MAF nuclei counterstained with DAPI. E: Lef1 and Tcf4 detection by Western analysis of nuclear extracts of fibroblast lines from 2 progeric patients (Coriell #AG11498, AG06297). F: Immunofluorescence showing Lef1 in normal parental (WT-AG03512) and Progeric (AG11498) fibroblasts, nuclei counterstained with DAPI.
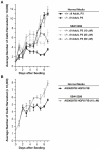
Figure 7. Cell Proliferation is enhanced by Gsk-3β inhibition
A: Treatment of Δ9MAFs with the Gsk inhibitor SB415286 (25μm) rescues growth. B: Treatment of the progeric line AG06297 (10μm SB415286) enhances growth.
Comment in
- Signaling defects and the nuclear envelope in progeria.
Muchir A, Worman HJ. Muchir A, et al. Dev Cell. 2010 Sep 14;19(3):355-6. doi: 10.1016/j.devcel.2010.08.019. Dev Cell. 2010. PMID: 20833355
Similar articles
- Defective lamin A-Rb signaling in Hutchinson-Gilford Progeria Syndrome and reversal by farnesyltransferase inhibition.
Marji J, O'Donoghue SI, McClintock D, Satagopam VP, Schneider R, Ratner D, Worman HJ, Gordon LB, Djabali K. Marji J, et al. PLoS One. 2010 Jun 15;5(6):e11132. doi: 10.1371/journal.pone.0011132. PLoS One. 2010. PMID: 20559568 Free PMC article. - Identification of mitochondrial dysfunction in Hutchinson-Gilford progeria syndrome through use of stable isotope labeling with amino acids in cell culture.
Rivera-Torres J, Acín-Perez R, Cabezas-Sánchez P, Osorio FG, Gonzalez-Gómez C, Megias D, Cámara C, López-Otín C, Enríquez JA, Luque-García JL, Andrés V. Rivera-Torres J, et al. J Proteomics. 2013 Oct 8;91:466-77. doi: 10.1016/j.jprot.2013.08.008. Epub 2013 Aug 20. J Proteomics. 2013. PMID: 23969228 - Vitamin D receptor signaling improves Hutchinson-Gilford progeria syndrome cellular phenotypes.
Kreienkamp R, Croke M, Neumann MA, Bedia-Diaz G, Graziano S, Dusso A, Dorsett D, Carlberg C, Gonzalo S. Kreienkamp R, et al. Oncotarget. 2016 May 24;7(21):30018-31. doi: 10.18632/oncotarget.9065. Oncotarget. 2016. PMID: 27145372 Free PMC article. - A-type lamins and Hutchinson-Gilford progeria syndrome: pathogenesis and therapy.
Gonzalez JM, Pla D, Perez-Sala D, Andres V. Gonzalez JM, et al. Front Biosci (Schol Ed). 2011 Jun 1;3(3):1133-46. doi: 10.2741/216. Front Biosci (Schol Ed). 2011. PMID: 21622261 Review. - Towards delineating the chain of events that cause premature senescence in the accelerated aging syndrome Hutchinson-Gilford progeria (HGPS).
Dreesen O. Dreesen O. Biochem Soc Trans. 2020 Jun 30;48(3):981-991. doi: 10.1042/BST20190882. Biochem Soc Trans. 2020. PMID: 32539085 Free PMC article. Review.
Cited by
- Sp1 transcription factor interaction with accumulated prelamin a impairs adipose lineage differentiation in human mesenchymal stem cells: essential role of sp1 in the integrity of lipid vesicles.
Ruiz de Eguino G, Infante A, Schlangen K, Aransay AM, Fullaondo A, Soriano M, García-Verdugo JM, Martín AG, Rodríguez CI. Ruiz de Eguino G, et al. Stem Cells Transl Med. 2012 Apr;1(4):309-21. doi: 10.5966/sctm.2011-0010. Epub 2012 Apr 2. Stem Cells Transl Med. 2012. PMID: 23197810 Free PMC article. - Chromatin states and nuclear organization in development--a view from the nuclear lamina.
Mattout A, Cabianca DS, Gasser SM. Mattout A, et al. Genome Biol. 2015 Aug 25;16(1):174. doi: 10.1186/s13059-015-0747-5. Genome Biol. 2015. PMID: 26303512 Free PMC article. Review. - Accumulation of the inner nuclear envelope protein Sun1 is pathogenic in progeric and dystrophic laminopathies.
Chen CY, Chi YH, Mutalif RA, Starost MF, Myers TG, Anderson SA, Stewart CL, Jeang KT. Chen CY, et al. Cell. 2012 Apr 27;149(3):565-77. doi: 10.1016/j.cell.2012.01.059. Cell. 2012. PMID: 22541428 Free PMC article. - Lamina-associated polypeptide (LAP)2α and nucleoplasmic lamins in adult stem cell regulation and disease.
Gesson K, Vidak S, Foisner R. Gesson K, et al. Semin Cell Dev Biol. 2014 May;29(100):116-24. doi: 10.1016/j.semcdb.2013.12.009. Epub 2013 Dec 25. Semin Cell Dev Biol. 2014. PMID: 24374133 Free PMC article. Review. - Endothelial progerin expression causes cardiovascular pathology through an impaired mechanoresponse.
Osmanagic-Myers S, Kiss A, Manakanatas C, Hamza O, Sedlmayer F, Szabo PL, Fischer I, Fichtinger P, Podesser BK, Eriksson M, Foisner R. Osmanagic-Myers S, et al. J Clin Invest. 2019 Feb 1;129(2):531-545. doi: 10.1172/JCI121297. Epub 2018 Dec 18. J Clin Invest. 2019. PMID: 30422822 Free PMC article.
References
- Adams JC, Watt FM. Regulation of development and differentiation by the extracellular matrix. Development. 1993;117:1183–1198. - PubMed
- Almeida M, Han L, Bellido T, Manolagas SC, Kousteni S. Wnt proteins prevent apoptosis of both uncommitted osteoblast progenitors and differentiated osteoblasts by beta-catenin-dependent and -independent signaling cascades involving Src/ERK and phosphatidylinositol 3-kinase/AKT. J Biol Chem. 2005;280:41342–41351. - PubMed
- Ameye L, Young MF. Mice deficient in small leucine-rich proteoglycans: novel in vivo models for osteoporosis, osteoarthritis, Ehlers-Danlos syndrome, muscular dystrophy, and corneal diseases. Glycobiology. 2002;12:107R–116R. - PubMed
- Aszodi A, Legate KR, Nakchbandi I, Fassler R. What mouse mutants teach us about extracellular matrix function. Annu Rev Cell Dev Biol. 2006;22:591–621. - PubMed
- Behrens J, von Kries JP, Kuhl M, Bruhn L, Wedlich D, Grosschedl R, Birchmeier W. Functional interaction of beta-catenin with the transcription factor LEF-1. Nature. 1996;382:638–642. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AG035626/AG/NIA NIH HHS/United States
- R01 HD057873/HD/NICHD NIH HHS/United States
- Z01 BC005093/ImNIH/Intramural NIH HHS/United States
- R01HD057873/HD/NICHD NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases