Insight into the mechanism of human herpesvirus 7 U21-mediated diversion of class I MHC molecules to lysosomes - PubMed (original) (raw)
Insight into the mechanism of human herpesvirus 7 U21-mediated diversion of class I MHC molecules to lysosomes
Nicole L Glosson et al. J Biol Chem. 2010.
Abstract
The U21 open reading frame from human herpesvirus-7 encodes a membrane protein that associates with and redirects class I MHC molecules to the lysosomal compartment. The mechanism by which U21 accomplishes this trafficking excursion is unknown. Here we have examined the contribution of localization, glycosylation, domain structure, and the absence of substrate class I MHC molecules on the ability of U21 to traffic to lysosomes. Our results suggest the existence of a cellular protein necessary for U21-mediated rerouting of class I MHC molecules.
Figures
FIGURE 1.
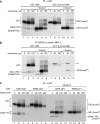
W6/32 coimmunoprecipitates soluble U21NSBPHA-tagged molecules. a, hypothetical schematic depiction of U21 and class I MHC binding and depiction of the soluble U21NSBPHA molecule. b, U373, U21, and U21NSBPHA cells were labeled with [35S]methionine for 90 min, lysed, and recovered with W6/32, directed against properly folded class I MHC molecules. Migration positions of U21, U21NSBPHA, and class I heavy chain (HC) molecules are indicated. Note that this figure depicts a hypothetical interaction between U21 and class I molecules; interaction domains, if any, are unknown. IP, immunoprecipitation.
FIGURE 2.
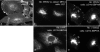
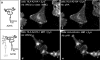
Soluble U21NSBPHA diverts class I molecules intracellularly. Immunofluorescent detection of class I MHC (W6/32) (panels a, b, and c), U21NSBPHA (panel d), and lamp2 (panel e). The arrows denote points of colocalization.
FIGURE 3.
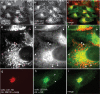
Localization of U21 in HeLa cells. a and b, HeLa cells infected with a U21-encoding puromycin-resistant lentivirus were double-labeled with W6/32 (anti-class I MHC) (a) and anti-U21 (b) prior to selection in puromycin. White arrowheads in b and e denote puncta barely visible in 1–2% of cells labeled with anti-U21. Pink arrows in a and d denote corresponding puncta visible with W6/32. c and f, merged images. d–f, enlarged images of the bottom cell in a–c, with arrowheads. g and h, U373 cells stably expressing HA-tagged U21 were double labeled with GM130, a marker of the _cis_-Golgi (g), and an anti-HA antibody (12CA5) (h). i, merged image. The white arrowheads in h denote the nuclear envelope, which is contiguous with the ER. Ab, antibody.
FIGURE 4.
U21NSBPHA-KDEL retains class I molecules in the ER. a, U373 or U21NSBPHA-KDEL cells were pulsed with [35S]methionine for 10 min and chased for the indicated times. Class I molecules were recovered with W6/32, digested with EndoH, and resolved by SDS-PAGE. The EndoH-sensitive and -resistant forms of the class I heavy chain (HC) and U21NSBPHA-KDEL are indicated. b, HA-tagged U21 was recovered from U21NSBPHA and U21NSBPHA-KDEL cells digested with EndoH and resolved by SDS-PAGE. The asterisks denote a nonspecific contaminating polypeptide.
FIGURE 5.
Class I MHC molecules in U21-KDEL cells are retained in the ER. Top panels, control U373 cells, U21-, U21NSBPHA-, and U21NSBPHA-KDEL-expressing cells were immunolabeled with W6/32 to examine class I localization. Bottom panels, for comparison, these cells were labeled with anti-protein-disulfide isomerase (PDI) as a marker for ER immunolabeling. Ab, antibody.
FIGURE 6.
Cells deficient in β2m lack W6/32 reactivity. Immunofluorescent detection of class I MHC molecules in U21-expressing (a) or U21 + shβ2m (b) U373 astrocytoma cells with the conformation-specific W6/32 antibody.
FIGURE 7.
EndoH sensitivity of U21 is unchanged when folded class I molecules are unavailable. U21-expressing or U21 + shβ2m (β_2m kd_) U373 astrocytoma cells were pulsed with [35S]methionine for 15 min and chased for the indicated times. Immunoprecipitates (IP) with either anti-U21 (a) or W6/32 (b) were divided, and half of the recovered material was treated with endoglycosidase H, as noted. U21 and class I heavy chains (HC) are noted, as are migration of EndoH-sensitive and EndoH-resistant forms of U21. Of note, EndoH-sensitive U21, which loses four _N_-linked glycans, migrates slightly more slowly than EndoH-sensitive class I MHC, so that the two bands are indistinguishable from one another (lanes 4). The double asterisk in b denotes a possible contaminating cross-reactive polypeptide (visible in lanes 5, 7, 8, 10, and 11). c, U21-expressing U373 or K562 cells were pulsed and chased and treated with EndoH as described above. U21, class I MHC heavy chains, and their EndoH-sensitive and -resistant counterparts are noted.
FIGURE 8.
Tailless HLA-A2 molecules are rerouted by U21. a, cartoon depicting the HLA-A2-TMHA fused molecules expressed in U373 cells in the absence or presence of U21. The HLA-A2 molecules consist of the transmembrane region of HLA-A2 and the membrane-proximal RRKSS sequence, followed by an HA tag. b–e, immunofluorescent detection of endogenous and HA-tagged class I MHC molecules with W6/32 (b and d) and an anti-HA polyclonal antibody (HA.11) (c and e). The cells and antibodies are noted. Ab, antibody.
FIGURE 9.
Tailless HLA-A2 molecules associate with U21. U373 or U373 cells expressing U21, A2HA, or A2HA and U21 were pulsed with [35S]methionine for 15 min and chased for 0 and 40 min, as noted. Class I molecules were recovered with W6/32 or 12CA5 (anti-HA). U21 molecules were recovered with MCW50. The migration positions of U21, class I heavy chain molecules (HC), and the chimeric tailless HLA-A2HA molecules are indicated. The asterisk (lanes 17 and 18) denotes a possible breakdown product present only in the U21 cells. Ab, antibody; IP, immunoprecipitation.
FIGURE 10.
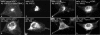
U21 reroutes H-2Kb-HLA-A2-tail 3K3R chimeric molecules. a, cartoon depicting the H-2Kb-HLA-A2-tail 3K→3R chimeric molecules expressed in U373 cells in the absence or presence of U21. b–g, U373 cells expressing H-2Kb-A2tail-3K3R without (b–d) or with (e–g) U21 were labeled with Y3 anti-H-2Kb tail mAb and the anti-U21 polyclonal antibody MCW50, as indicated. The merged images are shown in d and g. Ab, antibody.
FIGURE 11.
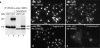
Tunicamycin interferes with U21-mediated diversion of class I molecules to lysosomes. a, U373 cells or U373 cells expressing U21 were labeled with [35S]methionine for 15 min in the absence or presence of tunicamycin, as noted. Class I MHC molecules were recovered with W6/32. The glycosylated and nonglycosylated class I heavy chain (HC) and U21 molecules are noted. b–e, low magnification U21 cells immunolabeled with anti-lamp2 (b and d) or class I MHC (W6/32) (c and e) after 6 h in the absence (b and c) or presence (d and e) of tunicamycin. Ab, antibody; IP, immunoprecipitation.
FIGURE 12.
Mutation of the _N_-linked glycosylation consensus site in HHV6-U21 affects U21-mediated rerouting of class I molecules to lysosomes. a–d, double-label immunolocalization of class I MHC molecules using W6/32 in cells expressing HHV-6A U21 or HHV6A U21 with its sole _N_-linked glycosylation site mutated to glutamine (N164Q), as noted. The SBPHA-tagged HHV6 U21 molecules are labeled with an anti-HA Ab (HA.11). e and f, flow cytometric analysis to detect surface class I MHC molecules in U373 cells (black traces) and U373 cells expressing HHV6 U21 or HHV6 U21 Asn → Gln (gray traces), as noted. The dotted traces in e and f represent cells incubated in the absence of phosphatidylethanolamine-conjugated anti-HLA-A, -B, or -C. Ab, antibody.
FIGURE 13.
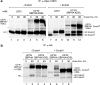
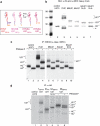
The HHV6A U21 glycosylation site mutants are expressed and can associate with class I MHC molecules. a, cartoon depicting the different U21 chimeric molecules expressed to assess the potential role of N_-linked glycans. U21 molecules are as noted, in red (HHV7), pink (HHV6B), and magenta (HHV6A). The U21HA molecule consists of full-length HHV6A U21 with an appended C-terminal HA tag. All other molecules have their cytoplasmic tails replaced with an SBPHA tag, as depicted. b, immunoblot of cells expressing HHV-7, HHV6B, and HHV6A U21 molecules, as well as HHV6A U21 glycosylation mutants. All of the cells express the chimeric U21SBPHA versions of U21, but they are not noted in the figure because of space constraints. The membrane was probed with anti-HA and anti-class I MHC heavy chain (HC10). The migration position of HHV-7 U21 with four N_-linked glycans (U21+4) is noted, as is the position of nonglycosylated U21 (U210) and the singly glycosylated class I MHC heavy chain (HC+1). The asterisk denotes a likely proteolytic fragment of HHV-7 U21SBPHA that is reactive with the HA.11 Ab (lane 3). The molecular weight standards are shown in lane 1. c, immunoprecipitation (IP) of class I MHC molecules from [35S]methionine-labeled cells (40-min pulse) expressing HHV-7, HHV6B, and HHV6A U21 molecules, as well as HHV6A U21 glycosylation mutants. All of the cells express the chimeric U21SBPHA versions of U21, but they are not noted in the figure because of space constraints. The migration position of HHV-7 U21 with four _N_-linked glycans (U21+4) is noted, as is the position of nonglycosylated U21 (U210) and the singly glycosylated class I MHC heavy chain (HC+1). Class I MHC molecules were recovered from cells with W6/32, and half of the immunoprecipitates were digested with PNGase:F and resolved by SDS-PAGE. The migration position of deglycosylated class I heavy chains (HC0) and U21 (U210) is indicated. d, immunoprecipitation of HA-tagged U21 from [35S]methionine-labeled cells (40-min pulse) expressing HHV-7, HHV6B, and HHV6A U21 molecules, as well as HHV6A U21 glycosylation mutants and HHV6AHA molecules. For HHV6B U21SBPHA cells, half of the immunoprecipitates were subject to PNGase treatment, as described above (lanes 5 and 6). The migration positions of U21 and class I heavy chains are noted, as in b and c.
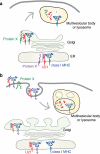
FIGURE 14.
Models depicting U21-mediated diversion of class I MHC molecules to the lysosomal compartment. U21 (red) associates with and diverts class I MHC molecules (blue) to a lamp1- and lamp2-positive lysosomal compartment. In a, U21-class I MHC complex associates with a cellular protein, Protein X (green or purple), which contains lysosomal targeting information. The association with Protein X could occur in the ER (purple) or later on in the biosynthetic pathway (green), accounting for a short-lived association with U21, and the lack of apparent coimmunoprecipitation of U21 with Protein X. Trafficking to lysosomes may involve a direct route from the Golgi to the lysosomal compartment or may involve arrival at the plasma membrane and subsequent routing through endosomes (depicted in b). In b, U21 (red) associates with class I MHC molecules and causes a conformational change in the class I molecule that is transduced through the membrane to the cytoplasmic tail. Through its lumenal association with U21, the cytoplasmic tail of the class I MHC molecule is somehow rendered competent to be modified by a Protein X (green), which then targets the complex of U21 and class I to an endolysosomal compartment.
Similar articles
- HHV-7 U21 exploits Golgi quality control carriers to reroute class I MHC molecules to lysosomes.
Dirck AT, Whyte ML, Hudson AW. Dirck AT, et al. Mol Biol Cell. 2020 Feb 1;31(3):196-208. doi: 10.1091/mbc.E19-07-0363. Epub 2019 Dec 18. Mol Biol Cell. 2020. PMID: 31851583 Free PMC article. - Adaptor protein complexes AP-1 and AP-3 are required by the HHV-7 Immunoevasin U21 for rerouting of class I MHC molecules to the lysosomal compartment.
Kimpler LA, Glosson NL, Downs D, Gonyo P, May NA, Hudson AW. Kimpler LA, et al. PLoS One. 2014 Jun 5;9(6):e99139. doi: 10.1371/journal.pone.0099139. eCollection 2014. PLoS One. 2014. PMID: 24901711 Free PMC article. - The ER-lumenal domain of the HHV-7 immunoevasin U21 directs class I MHC molecules to lysosomes.
Hudson AW, Blom D, Howley PM, Ploegh HL. Hudson AW, et al. Traffic. 2003 Dec;4(12):824-37. doi: 10.1046/j.1398-9219.2003.0137.x. Traffic. 2003. PMID: 14617346 - Antigen processing for presentation by MHC class I molecules.
Braciale TJ. Braciale TJ. Curr Opin Immunol. 1992 Feb;4(1):59-62. doi: 10.1016/0952-7915(92)90126-y. Curr Opin Immunol. 1992. PMID: 1596368 Review. - Structural Models for Roseolovirus U20 And U21: Non-Classical MHC-I Like Proteins From HHV-6A, HHV-6B, and HHV-7.
Weaver GC, Arya R, Schneider CL, Hudson AW, Stern LJ. Weaver GC, et al. Front Immunol. 2022 Apr 4;13:864898. doi: 10.3389/fimmu.2022.864898. eCollection 2022. Front Immunol. 2022. PMID: 35444636 Free PMC article. Review.
Cited by
- Human herpesviridae methods of natural killer cell evasion.
Odom CI, Gaston DC, Markert JM, Cassady KA. Odom CI, et al. Adv Virol. 2012;2012:359869. doi: 10.1155/2012/359869. Epub 2012 Jul 8. Adv Virol. 2012. PMID: 22829821 Free PMC article. - Viral inhibition of the transporter associated with antigen processing (TAP): a striking example of functional convergent evolution.
Verweij MC, Horst D, Griffin BD, Luteijn RD, Davison AJ, Ressing ME, Wiertz EJ. Verweij MC, et al. PLoS Pathog. 2015 Apr 16;11(4):e1004743. doi: 10.1371/journal.ppat.1004743. eCollection 2015 Apr. PLoS Pathog. 2015. PMID: 25880312 Free PMC article. Review. - Update on human herpesvirus 7 pathogenesis and clinical aspects as a roadmap for future research.
Verbeek R, Vandekerckhove L, Van Cleemput J. Verbeek R, et al. J Virol. 2024 Jun 13;98(6):e0043724. doi: 10.1128/jvi.00437-24. Epub 2024 May 8. J Virol. 2024. PMID: 38717112 Free PMC article. Review. - N-glycosylation of asparagine 8 regulates surface expression of major histocompatibility complex class I chain-related protein A (MICA) alleles dependent on threonine 24.
Mellergaard M, Skovbakke SL, Schneider CL, Lauridsen F, Andresen L, Jensen H, Skov S. Mellergaard M, et al. J Biol Chem. 2014 Jul 18;289(29):20078-91. doi: 10.1074/jbc.M114.573238. Epub 2014 May 28. J Biol Chem. 2014. PMID: 24872415 Free PMC article. - HHV-7 U21 exploits Golgi quality control carriers to reroute class I MHC molecules to lysosomes.
Dirck AT, Whyte ML, Hudson AW. Dirck AT, et al. Mol Biol Cell. 2020 Feb 1;31(3):196-208. doi: 10.1091/mbc.E19-07-0363. Epub 2019 Dec 18. Mol Biol Cell. 2020. PMID: 31851583 Free PMC article.
References
- Dewhurst S., Skrincosky D., van Loon N. (1997) Expert Rev. Mol. Med. 1997, 1–17 - PubMed
- Zerr D. M. (2006) Herpes 13, 20–24 - PubMed
- Ward K. N. (2005) Curr. Opin. Infect. Dis. 18, 247–252 - PubMed
- Tortorella D., Gewurz B. E., Furman M. H., Schust D. J., Ploegh H. L. (2000) Annu Rev. Immunol. 18, 861–926 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials