Acceptability and accuracy of a non-endoscopic screening test for Barrett's oesophagus in primary care: cohort study - PubMed (original) (raw)
Comparative Study
doi: 10.1136/bmj.c4372.
Pierre Lao-Sirieix, Maria O'Donovan, Irene Debiram, Madhumita Das, Jane M Blazeby, Jon Emery, Alex Boussioutas, Helen Morris, Fiona M Walter, Paul Pharoah, Richard H Hardwick, Rebecca C Fitzgerald
Affiliations
- PMID: 20833740
- PMCID: PMC2938899
- DOI: 10.1136/bmj.c4372
Comparative Study
Acceptability and accuracy of a non-endoscopic screening test for Barrett's oesophagus in primary care: cohort study
Sudarshan R Kadri et al. BMJ. 2010.
Abstract
Objectives: To determine the accuracy and acceptability to patients of non-endoscopic screening for Barrett's oesophagus, using an ingestible oesophageal sampling device (Cytosponge) coupled with immunocytochemisty for trefoil factor 3.
Design: Prospective cohort study.
Setting: 12 UK general practices, with gastroscopies carried out in one hospital endoscopy unit.
Participants: 504 of 2696 eligible patients (18.7%) aged 50 to 70 years with a previous prescription for an acid suppressant (H(2) receptor antagonist or proton pump inhibitor) for more than three months in the past five years.
Main outcome measures: Sensitivity and specificity estimates for detecting Barrett's oesophagus compared with gastroscopy as the ideal method, and patient anxiety (short form Spielberger state trait anxiety inventory, impact of events scale) and acceptability (visual analogue scale) of the test.
Results: 501 of 504 (99%) participants (median age 62, male to female ratio 1:1.2) successfully swallowed the Cytosponge. No serious adverse events occurred. In total, 3.0% (15/501) had an endoscopic diagnosis of Barrett's oesophagus (≥1 cm circumferential length, median circumferential and maximal length of 2 cm and 5 cm, respectively) with intestinal metaplasia. Compared with gastroscopy the sensitivity and specificity of the test was 73.3% (95% confidence interval 44.9% to 92.2%) and 93.8% (91.3% to 95.8%) for 1 cm or more circumferential length and 90.0% (55.5% to 99.7%) and 93.5% (90.9% to 95.5%) for clinically relevant segments of 2 cm or more. Most participants (355/496, 82%, 95% confidence interval 78.9% to 85.1%) reported low levels of anxiety before the test, and scores remained within normal limits at follow-up. Less than 4.5% (2.8% to 6.1%) of participants reported psychological distress a week after the procedure.
Conclusions: The performance of the Cytosponge test was promising and the procedure was well tolerated. These data bring screening for Barrett's oesophagus into the realm of possibility. Further evaluation is recommended.
Conflict of interest statement
Competing interests: All authors have completed the Unified Competing Interest form at www.icmje.org/coi\_disclosure.pdf (available on request from the corresponding author) and declare that SRK, PLS, MO’D, ID, MD, JMB, JE, AB, HM, FWM, RHH, and RCF have no relationships with companies that might have an interest in the submitted work in the previous 3 years; their spouses, partners, or children have no financial relationships that may be relevant to the submitted work; and SRK, PLS, MO’D, ID, MD, JMB, JE, AB, HM, FWM, RHH, and RCF have no non-financial interests that may be relevant to the submitted work.
Figures
Fig 1 Cytosponge in gelatine capsule (right) and expanded (left)
Fig 2 Flow of patients through trial
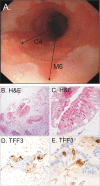
Fig 3 Endoscopic view of a patient with C2M4 segment length Barrett’s oesophagus (panel A). Haematoxylin and eosin (panel B ×100, panel C ×400) and trefoil factor 3 (panel D ×100, panel E ×400) staining from representative patient with Barrett’s oesophagus
Fig 4 Participants’ experience, anxiety, and distress related to Cytosponge test. Anxiety measured at day 0, 7, and 90 using six item short form short form Spielberger state trait anxiety inventory (values >40 denote clinically significant anxiety). Distress associated with Cytosponge test measured using impact of events scale. Intrusion and avoidance scores >19 denote high impact of test and >38 for total score. Participants rated their experience of Cytosponge using a visual analogue scale, with 0 representing the “worst experience” and 10 the “best experience”
Comment in
- Non-endoscopic screening for Barrett's oesophagus.
Bampton PA. Bampton PA. BMJ. 2010 Sep 10;341:c4667. doi: 10.1136/bmj.c4667. BMJ. 2010. PMID: 20833744 No abstract available. - Cytosponge for Barrett's esophagus screening: when smart science matches simplicity.
Triadifilopoulos G. Triadifilopoulos G. Gastroenterology. 2011 Aug;141(2):766-8; discussion 768. doi: 10.1053/j.gastro.2011.06.012. Epub 2011 Jun 23. Gastroenterology. 2011. PMID: 21704039 No abstract available.
Similar articles
- Cytosponge-trefoil factor 3 versus usual care to identify Barrett's oesophagus in a primary care setting: a multicentre, pragmatic, randomised controlled trial.
Fitzgerald RC, di Pietro M, O'Donovan M, Maroni R, Muldrew B, Debiram-Beecham I, Gehrung M, Offman J, Tripathi M, Smith SG, Aigret B, Walter FM, Rubin G; BEST3 Trial team; Sasieni P. Fitzgerald RC, et al. Lancet. 2020 Aug 1;396(10247):333-344. doi: 10.1016/S0140-6736(20)31099-0. Lancet. 2020. PMID: 32738955 Free PMC article. Clinical Trial. - Evaluation of a minimally invasive cell sampling device coupled with assessment of trefoil factor 3 expression for diagnosing Barrett's esophagus: a multi-center case-control study.
Ross-Innes CS, Debiram-Beecham I, O'Donovan M, Walker E, Varghese S, Lao-Sirieix P, Lovat L, Griffin M, Ragunath K, Haidry R, Sami SS, Kaye P, Novelli M, Disep B, Ostler R, Aigret B, North BV, Bhandari P, Haycock A, Morris D, Attwood S, Dhar A, Rees C, Rutter MD, Sasieni PD, Fitzgerald RC; BEST2 Study Group. Ross-Innes CS, et al. PLoS Med. 2015 Jan 29;12(1):e1001780. doi: 10.1371/journal.pmed.1001780. eCollection 2015 Jan. PLoS Med. 2015. PMID: 25634542 Free PMC article. - Use of a Cytosponge biomarker panel to prioritise endoscopic Barrett's oesophagus surveillance: a cross-sectional study followed by a real-world prospective pilot.
Pilonis ND, Killcoyne S, Tan WK, O'Donovan M, Malhotra S, Tripathi M, Miremadi A, Debiram-Beecham I, Evans T, Phillips R, Morris DL, Vickery C, Harrison J, di Pietro M, Ortiz-Fernandez-Sordo J, Haidry R, Kerridge A, Sasieni PD, Fitzgerald RC. Pilonis ND, et al. Lancet Oncol. 2022 Feb;23(2):270-278. doi: 10.1016/S1470-2045(21)00667-7. Epub 2022 Jan 11. Lancet Oncol. 2022. PMID: 35030332 Free PMC article. - Surveillance of Barrett's oesophagus: exploring the uncertainty through systematic review, expert workshop and economic modelling.
Garside R, Pitt M, Somerville M, Stein K, Price A, Gilbert N. Garside R, et al. Health Technol Assess. 2006 Mar;10(8):1-142, iii-iv. doi: 10.3310/hta10080. Health Technol Assess. 2006. PMID: 16545207 Review. - Diagnosis and Management of Low-Grade Dysplasia in Barrett's Esophagus: Expert Review From the Clinical Practice Updates Committee of the American Gastroenterological Association.
Wani S, Rubenstein JH, Vieth M, Bergman J. Wani S, et al. Gastroenterology. 2016 Nov;151(5):822-835. doi: 10.1053/j.gastro.2016.09.040. Epub 2016 Oct 1. Gastroenterology. 2016. PMID: 27702561 Review.
Cited by
- Novel Screening Alternatives for Barrett Esophagus.
Krishna Chandar A, Sharma A, Chak A. Krishna Chandar A, et al. Gastroenterol Hepatol (N Y). 2020 May;16(5):238-245. Gastroenterol Hepatol (N Y). 2020. PMID: 34035726 Free PMC article. - Systematic review and meta-analysis of immunohistochemical prognostic biomarkers in resected oesophageal adenocarcinoma.
McCormick Matthews LH, Noble F, Tod J, Jaynes E, Harris S, Primrose JN, Ottensmeier C, Thomas GJ, Underwood TJ. McCormick Matthews LH, et al. Br J Cancer. 2015 Jun 30;113(1):107-18. doi: 10.1038/bjc.2015.179. Epub 2015 Jun 25. Br J Cancer. 2015. PMID: 26110972 Free PMC article. Review. - Recent developments in pathogenesis, diagnosis and therapy of Barrett's esophagus.
Halland M, Katzka D, Iyer PG. Halland M, et al. World J Gastroenterol. 2015 Jun 7;21(21):6479-90. doi: 10.3748/wjg.v21.i21.6479. World J Gastroenterol. 2015. PMID: 26074687 Free PMC article. Review. - Management of Barrett's esophageal carcinoma.
Miyazaki T, Inose T, Tanaka N, Yokobori T, Suzuki S, Ozawa D, Sohda M, Nakajima M, Fukuchi M, Kato H, Kuwano H. Miyazaki T, et al. Surg Today. 2013 Apr;43(4):353-60. doi: 10.1007/s00595-012-0468-2. Epub 2013 Jan 3. Surg Today. 2013. PMID: 23283352 Review. - Computational pathology aids derivation of microRNA biomarker signals from Cytosponge samples.
Masqué-Soler N, Gehrung M, Kosmidou C, Li X, Diwan I, Rafferty C, Atabakhsh E, Markowetz F, Fitzgerald RC. Masqué-Soler N, et al. EBioMedicine. 2022 Feb;76:103814. doi: 10.1016/j.ebiom.2022.103814. Epub 2022 Jan 17. EBioMedicine. 2022. PMID: 35051729 Free PMC article.
References
- Shaheen NJ, Richter JE. Barrett’s oesophagus. Lancet 2009;373:850-61. - PubMed
- Yousef F, Cardwell C, Cantwell MM, Galway K, Johnston BT, Murray L. The incidence of esophageal cancer and high-grade dysplasia in Barrett’s esophagus: a systematic review and meta-analysis. Am J Epidemiol 2008;168:237-49. - PubMed
- Medical Research Council Oesophageal Cancer Working Group. Surgical resection with or without preoperative chemotherapy in oesophageal cancer: a randomised controlled trial. Lancet 2002;359:1727-33. - PubMed
- Shaheen NJ, Sharma P, Overholt BF, Lightdale CJ, Wolfsen HC, Sampliner RE, et al. A randomized, multicenter, sham-controlled trial of radiofrequency ablation (RFA) for subjects with Barrett’s esophagus (BE) containing dysplasia: interim results of the AIM Dysplasia Trial. Gastroenterology 2008;134:750-8.
Publication types
MeSH terms
Substances
Grants and funding
- MC_U105365007/MRC_/Medical Research Council/United Kingdom
- A10119/CRUK_/Cancer Research UK/United Kingdom
- 11021/CRUK_/Cancer Research UK/United Kingdom
- 10119/CRUK_/Cancer Research UK/United Kingdom
- G0601390/MRC_/Medical Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous