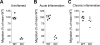Chemoattractant receptors and lymphocyte egress from extralymphoid tissue: changing requirements during the course of inflammation - PubMed (original) (raw)
Chemoattractant receptors and lymphocyte egress from extralymphoid tissue: changing requirements during the course of inflammation
Meghan N Brown et al. J Immunol. 2010.
Abstract
Memory/effector T cells traffic efficiently through extralymphoid tissues, entering from the blood and leaving via the afferent lymph. During inflammation, T cell traffic into the affected tissue dramatically increases; however, the dynamics and mechanisms of T cell exit from inflamed tissues are poorly characterized. In this study, we show, using both a mouse and a sheep model, that large numbers of lymphocytes leave the chronically inflamed skin. Many T cells capable of producing IFN-γ and IL-17 also entered the draining afferent lymph, demonstrating that memory/effector T cells egress from sites of inflammation. Whereas efficient egress from acutely inflamed skin required lymphocyte-expressed CCR7, chronic inflammation promoted significant CCR7-independent exit as well. Lymphocyte exit at late time points of inflammation was sensitive to pertussis toxin but was only partially affected by the drug FTY720, implying the contribution of alternative chemoattractant receptors other than spingosine 1-phosphate receptor 1. Our data show that CCR7 is an important receptor for lymphocyte egress from both resting and inflamed extralymphoid tissues, but that alternative exit receptors come into play during chronic inflammation.
Figures
Figure 1. Chronic inflammation enhances memory/effector T cell egress from the affected skin via the afferent lymph
Different stages of cutaneous inflammation were elicited by subcutaneous injection of CFA emulsified with saline into sheep flanks. Ovine peripheral blood or lymph was collected after venipuncture or catheterization of afferent lymph vessels draining uninflamed (control), acutely (≤48h after induction of inflammation) or chronically (3-5 weeks after induction of inflammation) inflamed skin. (A) Number of cells collected from the skin draining afferent lymph vessel over time (cell output) was determined for total cells, total lymphocytes, and CD4 and CD8 T cells. Data points show the mean ± SD of multiple time points analyzed for cell output from acutely inflamed and control (uninflamed) skin, and one time point for cell output from chronically inflamed skin of one animal. Data are representative of one animal out of a minimum of four individually analyzed sheep per time point of inflammation. (B) Flow cytometric analysis of L-selectin or E-selectin ligand expression by gated CD4 (top row) and CD8 (bottom row) T cells from afferent lymph draining chronically inflamed skin. Gray areas indicate isotype control staining. Numbers indicate the percent positive T cells in the specified gates. Data are representative of at least seven individually analyzed sheep. (C) Scatter plot of all analyzed animals, showing percentages of L-selectin (L-sel) and E-selectin ligand (E-lig) expressing CD4 and CD8 T cells in granuloma draining lymph. Data points represent individually analyzed sheep and horizontal lines indicate the mean of each group. (D) Intracellular cytokine staining for IFN-γ and IL-17 of gated afferent lymph CD4 and CD8 T cells draining chronically inflamed skin after stimulation with PMA and ionomycin. One representative staining of eight individually analyzed animals is shown. (E) Scatter plot of all analyzed animals, showing percentages of IFN-γ and IL-17 expressing CD4 and CD8 T cells in granuloma draining lymph. Data points represent individually analyzed sheep and horizontal lines indicate the mean of each group.
Figure 2. Chronic inflammation enhances lymphocyte egress from inflamed skin in an adoptive transfer mouse model
CFA was injected subcutaneously into the area of footpads of mice to induce cutaneous inflammation. At different time points of inflammation, histology was assessed by paraffin sections after H&E staining in control skin (A), acutely inflamed skin at 6h (B), and chronically inflamed skin 21d (C) after induction of inflammation. (D, E) CFSE-labeled splenic lymphocytes were transferred into acutely inflamed skin (D, open symbols) or chronically inflamed skin (E, open symbols) 6h and 21d after induction of inflammation, respectively, or into the skin of untreated control mice (closed symbols). 12h after transfer, migrated CFSE+ total lymphocytes, CD4 T cells, CD8 T cells, and B cells were enumerated in the draining popliteal lymph nodes by flow cytometry. Migration was expressed as the percent of injected cells of each respective lymphocyte subset that migrated to the draining lymph node. (A-C) One representative staining out of a minimum of 5 mice per condition is shown at 10x and 100x (insets) original magnification. Scale bars indicate 100μm and 5μm for insets (D, E). One representative out of a minimum of three experiments performed analyzing 8-10 mice per group is shown. Data points represent individually analyzed mice and horizontal lines indicate the mean of each group.
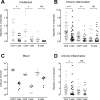
Figure 3. Chronic but not acute inflammation supports CCR7-indendent lymphocyte exit from inflamed skin
PKH26-labeled WT lymphocytes were mixed with equal numbers of either WT or CCR7-deficient CFSE-labeled lymphocytes and injected into the inflamed footpads of recipient mice 6h (A) or 10d (B) after induction of cutaneous inflammation with CFA. 12h after cell transfer, the draining popliteal lymph nodes were analyzed for migrated CFSE+ and PKH26+ cells. The ratio of migrated CFSE/PKH26 to injected CFSE/PKH26 cells was determined for total lymphocytes, total CD4 T cells, memory (CD45RBlo) CD4 T cells, naïve (CD45RBhi) CD4 T cells, total CD8 T cells and B cells. In each case, results were normalized to the mean ratio of WT CFSE+ cells to WT PKH26+ cells (internal standard cells) for each subset (set as 100%). Data points represent individually analyzed mice of groups of 4-5 mice; horizontal lines indicate the mean of each group. One representative of a minimum of three experiments analyzing each cell type is shown. WT, wild-type; KO, CCR7-deficient.
Figure 4. Th1 effector cell egress chronically inflamed is largely independent of CCR7
Th1 effector cells were generated in vitro from WT and CCR7-deficient mice. PKH26-labeled WT Th1 cells were mixed with equal numbers of either WT or CCR7-deficient CFSE-labeled Th1 cells and injected into the uninflamed (A), acutely inflamed (B; 6h after induction of inflammation with CFA), or chronically inflamed (C; 21d after induction of inflammation with CFA) footpad skin of recipient mice. 12h after cell transfer, the draining popliteal lymph nodes were analyzed for migrated CFSE+ and PKH26+ T cells. The ratio of migrated CFSE/PKH26 to the ratio injected CFSE/PKH26 Th1 cells was determined. Results were normalized to the mean ratio of WT CFSE+ cells to WT PKH26+ cells (internal standard cells), which was set as 100%. Data points represent individually analyzed mice in groups of 5 mice; horizontal lines indicate the mean of each group. One representative of a minimum of three experiments for each condition (A and C) or one out of two with similar results (B) is shown. WT, wild-type; KO, CCR7-deficient.
Figure 5. Lymphocyte egress from chronically inflamed skin depends on Gαi protein-coupled receptors signaling
Splenic lymphocytes were incubated with PTX or control treated and subsequently labeled with CFSE or PKH26, respectively. 21d after induction of cutaneous inflammation with CFA in both hind footpads, PTX-treated CFSE+ cells were injected into the inflamed footpad skin of one side and PKH26+ cells into the inflamed skin of the contralateral footpad. 12h after cell transfer, fluorescently labeled lymphocytes that migrated into the draining lymph node were enumerated by flow cytometry. One representative staining (A) and experiment (B) out of three performed experiments analyzing 9-10 mice each are shown.
Figure 6. FTY720 treatment reduces T cell egress from chronically inflamed skin
Naïve control mice (A) or mice with 21d-old CFA-induced cutaneous inflammation in both hind footpads (B-D) were treated intraperitoneally with FTY720 (closed symbols) or saline (open symbols). 8h post treatment, CFSE- and/or PKH26-labeled splenic lymphocytes from wild-type (A, B) or CCR7-deficient (D) mice were injected into the uninflamed (A) or inflamed (B, D) footpad skin of the FTY720-treated and control-treated mice. 12h after cell transfer, fluorescently labeled lymphocytes that migrated into the draining popliteal lymph node (A, B, D) and endogenous lymphocytes in blood (C) were enumerated by flow cytometry. Data points represent individually analyzed recipient mice and horizontal lines indicate the mean of each group. The combination of data from 2 experiments analyzing 5-10 mice per group (A), 5 experiments analyzing 8-10 mice per group (B), 3 experiments analyzing 10 mice per group (D), and one example of a corresponding endogenous blood lymphocyte count (C) is shown. *, p<0.05; **, p<0.01; ***, p< 0.001; NS, not significant (p>0.05).
Figure 7. Chronicity of inflammation determines the efficiency of CCR7-dependent and independent lymphocyte egress from extralymphoid tissue
Different stages of cutaneous inflammation were induced by subcutaneous injection of CFA into the footpads of mice at different time points. CCR7-deficient and WT splenocytes were labeled with CFSE and PKH26, respectively, mixed, and injected into inflamed footpad skin of mice at the different indicated time points of a CFA-induced inflammation or into footpads of untreated mice (Control). 12h after cell transfer, migrated CFSE+ and PKH26+ lymphocytes were enumerated in the draining popliteal lymph nodes. One out of two experiments with similar results, analyzing four to five mice per time point, is shown.
Similar articles
- CXCR4 is dispensable for T cell egress from chronically inflamed skin via the afferent lymph.
Geherin SA, Wilson RP, Jennrich S, Debes GF. Geherin SA, et al. PLoS One. 2014 Apr 21;9(4):e95626. doi: 10.1371/journal.pone.0095626. eCollection 2014. PLoS One. 2014. PMID: 24752354 Free PMC article. - Chemokine receptor CCR7 required for T lymphocyte exit from peripheral tissues.
Debes GF, Arnold CN, Young AJ, Krautwald S, Lipp M, Hay JB, Butcher EC. Debes GF, et al. Nat Immunol. 2005 Sep;6(9):889-94. doi: 10.1038/ni1238. Epub 2005 Aug 14. Nat Immunol. 2005. PMID: 16116468 Free PMC article. - Ovine skin-recirculating γδ T cells express IFN-γ and IL-17 and exit tissue independently of CCR7.
Geherin SA, Lee MH, Wilson RP, Debes GF. Geherin SA, et al. Vet Immunol Immunopathol. 2013 Sep 1;155(1-2):87-97. doi: 10.1016/j.vetimm.2013.06.008. Epub 2013 Jun 18. Vet Immunol Immunopathol. 2013. PMID: 23838472 Free PMC article. - Multifaceted activities of CCR7 regulate T-cell homeostasis in health and disease.
Moschovakis GL, Förster R. Moschovakis GL, et al. Eur J Immunol. 2012 Aug;42(8):1949-55. doi: 10.1002/eji.201242614. Eur J Immunol. 2012. PMID: 22700449 Review. - Chemokine-mediated control of T cell traffic in lymphoid and peripheral tissues.
Ebert LM, Schaerli P, Moser B. Ebert LM, et al. Mol Immunol. 2005 May;42(7):799-809. doi: 10.1016/j.molimm.2004.06.040. Epub 2004 Nov 23. Mol Immunol. 2005. PMID: 15829268 Review.
Cited by
- The skin, a novel niche for recirculating B cells.
Geherin SA, Fintushel SR, Lee MH, Wilson RP, Patel RT, Alt C, Young AJ, Hay JB, Debes GF. Geherin SA, et al. J Immunol. 2012 Jun 15;188(12):6027-35. doi: 10.4049/jimmunol.1102639. Epub 2012 May 4. J Immunol. 2012. PMID: 22561151 Free PMC article. - T Cell Trafficking through Lymphatic Vessels.
Hunter MC, Teijeira A, Halin C. Hunter MC, et al. Front Immunol. 2016 Dec 21;7:613. doi: 10.3389/fimmu.2016.00613. eCollection 2016. Front Immunol. 2016. PMID: 28066423 Free PMC article. Review. - CCL8 and skin T cells--an allergic attraction.
Debes GF, Diehl MC. Debes GF, et al. Nat Immunol. 2011 Feb;12(2):111-2. doi: 10.1038/ni0211-111. Nat Immunol. 2011. PMID: 21245898 No abstract available. - Lymphatic Vessels, Inflammation, and Immunity in Skin Cancer.
Lund AW, Medler TR, Leachman SA, Coussens LM. Lund AW, et al. Cancer Discov. 2016 Jan;6(1):22-35. doi: 10.1158/2159-8290.CD-15-0023. Epub 2015 Nov 9. Cancer Discov. 2016. PMID: 26552413 Free PMC article. Review. - Defective T cell chemotaxis to sphingosine 1-phosphate and chemokine CCL21 in idiopathic T lymphocytopenia.
Goetzl EJ, Schwartz JB, Huang MC. Goetzl EJ, et al. J Clin Immunol. 2011 Oct;31(5):744-51. doi: 10.1007/s10875-011-9554-2. Epub 2011 Jun 14. J Clin Immunol. 2011. PMID: 21671128
References
- Campbell DJ, Debes GF, Johnston B, Wilson E, Butcher EC. Targeting T cell responses by selective chemokine receptor expression. Semin Immunol. 2003;15:277–286. - PubMed
- Luster AD, Alon R, von Andrian UH. Immune cell migration in inflammation: present and future therapeutic targets. Nat Immunol. 2005;6:1182–1190. - PubMed
- Chin W, Hay JB. A comparison of lymphocyte migration through intestinal lymph nodes, subcutaneous lymph nodes, and chronic inflammatory sites of sheep. Gastroenterology. 1980;79:1231–1242. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AR056730-02/AR/NIAMS NIH HHS/United States
- T32 AI007290/AI/NIAID NIH HHS/United States
- R01 AI072618/AI/NIAID NIH HHS/United States
- AI72618/AI/NIAID NIH HHS/United States
- T32 AI007532/AI/NIAID NIH HHS/United States
- AI47822/AI/NIAID NIH HHS/United States
- R21 AI047822/AI/NIAID NIH HHS/United States
- R01 AI047822/AI/NIAID NIH HHS/United States
- R00 AI073682-03/AI/NIAID NIH HHS/United States
- R37 AI047822/AI/NIAID NIH HHS/United States
- AI073682/AI/NIAID NIH HHS/United States
- R01 AR056730-01A1/AR/NIAMS NIH HHS/United States
- R00 AI073682/AI/NIAID NIH HHS/United States
- T32AI007532/AI/NIAID NIH HHS/United States
- AR056730/AR/NIAMS NIH HHS/United States
- K99 AI073682/AI/NIAID NIH HHS/United States
- R01 AR056730/AR/NIAMS NIH HHS/United States
- R00 AI073682-02/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases