Inhibition of O-GlcNAcase in perfused rat hearts by NAG-thiazolines at the time of reperfusion is cardioprotective in an O-GlcNAc-dependent manner - PubMed (original) (raw)
Inhibition of O-GlcNAcase in perfused rat hearts by NAG-thiazolines at the time of reperfusion is cardioprotective in an O-GlcNAc-dependent manner
Boglarka Laczy et al. Am J Physiol Heart Circ Physiol. 2010 Nov.
Abstract
Acute increases in O-linked β-N-acetylglucosamine (O-GlcNAc) levels of cardiac proteins exert protective effects against ischemia-reperfusion (I/R) injury. One strategy to rapidly increase cellular O-GlcNAc levels is inhibition of O-GlcNAcase (OGA), which catalyzes O-GlcNAc removal. Here we tested the cardioprotective efficacy of two novel and highly selective OGA inhibitors, the NAG-thiazoline derivatives NAG-Bt and NAG-Ae. Isolated perfused rat hearts were subjected to 20 min global ischemia followed by 60 min reperfusion. At the time of reperfusion, hearts were assigned to the following four groups: 1) untreated control; 2) 50 μM NAG-Bt; 3) 100 μM NAG-Bt; or 4) 50 μM NAG-Ae. All treatment groups significantly increased total O-GlcNAc levels (P < 0.05 vs. control), and this was significantly correlated with improved contractile function and reduced cardiac troponin I release (P < 0.05). Immunohistochemistry of normoxic hearts showed intense nuclear O-GlcNAc staining and higher intensity at Z-lines with colocalization of O-GlcNAc and the Z-line proteins desmin and vinculin. After I/R, there was a marked loss of both cytosolic and nuclear O-GlcNAcylation and disruption of normal striated Z-line structures. OGA inhibition largely preserved structural integrity and attenuated the loss of O-GlcNAcylation; however, nuclear O-GlcNAc levels remained low. Immunoblot analysis confirmed ∼50% loss in both nuclear and cytosolic O-GlcNAcylation following I/R, which was significantly attenuated by OGA inhibition (P < 0.05). These data provide further support for the notion that increasing cardiac O-GlcNAc levels by inhibiting OGA may be a clinically relevant approach for ischemic cardioprotection, in part, by preserving the integrity of O-GlcNAc-associated Z-line protein structures.
Figures
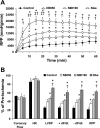
Fig. 1.
Effect of 1,2 dideoxy-2′-methyl-α-
d
-glycopyranoso-[2,1-d]-Δ2′-thiazoline (NAG-thiazoline) treatment during reperfusion on functional recovery after 20 min zero-flow ischemia. A: time course of changes in rate-pressure product (RPP) during 60 min reperfusion. B: functional recoveries of coronary flow, heart rate (HR), left ventricular developed pressure (LVDP), positive and negative rates of left ventricular pressure change (±dP/d_t_), and RPP following 60 min reperfusion as a percent of preischemic values in untreated ischemia-reperfusion hearts (control; n = 7) and hearts treated with 50 μM 1,2 dideoxy-2′-propyl-α-
d
-glycopyranoso-[2,1-d]-Δ2′-thiazoline [NAG-Bt (NBt50); n = 7], 100 μM NAG-Bt (NBt100; n = 5), and 50 μM 1,2 dideoxy-2′-ethylamino-α-
d
-glucopyranoso-[2,1-d]-Δ2′-thiazoline [NAG-Ae (NAe); n = 3]. P < 0.05 vs. control (*), vs. NBt50 (†), and vs. NBt100 (‡).
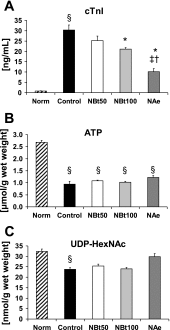
Fig. 2.
Effect of NAG-thiazoline treatment on cardiac troponin I (cTnI) release (A), ATP levels (B), and UDP-_N_-acetylhexosamine (HexNAc) concentrations (C) in untreated, time-control, normoxic hearts (Norm, n = 5) at the end of 110 min normoxic perfusion and at the end of 60 min reperfusion after 20 min zero-flow ischemia in untreated ischemia-reperfusion hearts (control, n = 7) and NBt50 (n = 7), NBt100 (n = 5), and NAe (n = 3) hearts. P < 0.05 vs. control (*), vs. NBt50 (†), vs. NBt100 (‡), and vs. Norm (§).
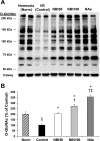
Fig. 3.
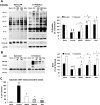
Effect of NAG-thiazoline treatment on _O_-linked β-_N_-acetylglucosamine (_O_-GlcNAc) levels of the perfused rat heart. Representative anti-_O_-GlcNAc (CTD110.6) immunoblot (A) and densitometric results of protein-associated cardiac _O_-GlcNAc levels (B) after time-control, normoxic perfusions (Norm); and after 20 min zero-flow ischemia and 60 min reperfusion (I/R) in the absence (control) and in the presence of NBt50, NBt100, and NAe treatments. Densitometric analyses were performed using original anti-_O_-GlcNAc immunoblots to compare all experimental groups (n = 3–5 hearts/group). The entire lane mean intensities are normalized to calsequestrin levels shown as protein loading control, and are relative to the control group. P < 0.001 vs. control (*), vs. NBt50 (†), vs. NBt100 (‡), and vs. Norm (§).
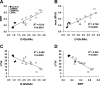
Fig. 4.
Correlations of cardiac O_-GlcNAc levels and RPP (A), maximum rate of left ventricular pressure changes (max dP/d_t; B), and cTnI release (C) at the end of reperfusion. D: correlation of cTnI levels and RPP after reperfusion. Data of cardiac functions are expressed as %preischemic values and are presented for all ischemia-reperfusion hearts in the untreated group (control; n = 7) and following treatments in the NBt50 (n = 7), NBt100 (n = 5), and NAe (n = 3) groups.
Fig. 5.
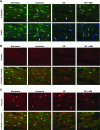
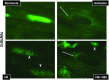
Immunohistochemistry of rat myocardium after normoxic perfusions (Normoxia); after 20 min zero-flow ischemia (Ischemia); at the end of 60 min reperfusion without treatment (I/R); and after reperfusion following treatment with 50 μM NAG-Bt (I/R + NBt). A: _O_-GlcNAc immunohistochemistry (green) and merged images with nuclei staining (blue; 4′,6-diamidino-2-phenylindole, DAPI); note that ischemia and I/R result in alterations in nuclear _O_-GlcNAc distribution with appearance of punctate staining (arrowheads) and perinuclear staining of otherwise _O_-GlcNAc-negative nuclei (arrows). B: desmin immunohistochemistry (red) and its cross-striated colocalization with _O_-GlcNAc (green) at Z-line. C: vinculin immunohistochemistry (red) and colocalization of _O_-GlcNAc (green) with vinculin at Z-line, but not intercalated disc (*) (×40 magnification).
Fig. 6.
Immunohistochemistry of nuclear _O_-GlcNAcylation in response to ischemia, I/R, I/R + NBt. In normoxic hearts (Normoxia), intensity of _O_-GlcNAc (green) staining is predominantly concentrated in the nuclei of cardiomyocytes. Ischemia leads to prominent changes in nuclear _O_-GlcNAc distribution with increased punctate staining (arrowheads) and perinuclear staining of otherwise _O_-GlcNAc-negative nuclei (arrows).
Fig. 7.
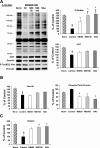
Effect of I/R and NAG-thiazoline treatment on _O_-GlcNAc levels and _O_-GlcNAc transferase (OGT) protein content in nuclear and cytosolic compartments of the perfused rat heart. A: representative anti-_O_-GlcNAc (CTD110.6) immunoblots, and densitometric analyses, demonstrate the changes in nuclear and cytosolic levels of _O_-GlcNAc. Note that, given the marked differences in overall _O_-GlcNAc levels between nuclear and cytosolic fractions, different exposure times as indicated were used for the gel images. Consequently, the mean data are shown normalized to the intensity of the untreated group [control (Ctr)] for each fraction; thus, comparison of _O_-GlcNAc levels between the two fractions cannot be made. B and C: OGT (B) and high-molecular-weight OGT (C) immunoreactive bands in the cytosolic fraction in hearts after time-control, normoxic perfusions (Norm) and after I/R in the untreated group (control) and the treated NBt50, NBt100, and NAe groups (n = 3–4 hearts/group). TATA-binding protein and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) are shown as purity controls. P < 0.05, nuclear vs. cytosolic (*), vs. cytosolic control (†), vs. nuclear control (‡), vs. cytosolic Norm (§), and vs. nuclear Norm (#).
Fig. 8.
Immunoblot analyses of cardiac _O_-GlcNAc and OGT (A), phospho-Tyr822 vinculin and total vinculin (B), and desmin (C) levels in the crude membrane fraction (n = 3 hearts/group) after time-control, normoxic perfusions (Norm) and after I/R in untreated hearts (control) and hearts from NBt50, NBt100, and NAe groups. Data are expressed as %control group. P < 0.05 vs. control (*), vs. NBt50 (†), and vs. Norm (§).
Similar articles
- Increased O-GlcNAc levels during reperfusion lead to improved functional recovery and reduced calpain proteolysis.
Liu J, Marchase RB, Chatham JC. Liu J, et al. Am J Physiol Heart Circ Physiol. 2007 Sep;293(3):H1391-9. doi: 10.1152/ajpheart.00285.2007. Epub 2007 Jun 15. Am J Physiol Heart Circ Physiol. 2007. PMID: 17573462 Free PMC article. - Glucosamine protects neonatal cardiomyocytes from ischemia-reperfusion injury via increased protein O-GlcNAc and increased mitochondrial Bcl-2.
Champattanachai V, Marchase RB, Chatham JC. Champattanachai V, et al. Am J Physiol Cell Physiol. 2008 Jun;294(6):C1509-20. doi: 10.1152/ajpcell.00456.2007. Epub 2008 Mar 26. Am J Physiol Cell Physiol. 2008. PMID: 18367586 Free PMC article. - O-GlcNAc signaling attenuates ER stress-induced cardiomyocyte death.
Ngoh GA, Hamid T, Prabhu SD, Jones SP. Ngoh GA, et al. Am J Physiol Heart Circ Physiol. 2009 Nov;297(5):H1711-9. doi: 10.1152/ajpheart.00553.2009. Epub 2009 Sep 4. Am J Physiol Heart Circ Physiol. 2009. PMID: 19734355 Free PMC article. - Increasing O-GlcNAc levels: An overview of small-molecule inhibitors of O-GlcNAcase.
Macauley MS, Vocadlo DJ. Macauley MS, et al. Biochim Biophys Acta. 2010 Feb;1800(2):107-21. doi: 10.1016/j.bbagen.2009.07.028. Epub 2009 Aug 4. Biochim Biophys Acta. 2010. PMID: 19664691 Review. - O-GlcNAcase: promiscuous hexosaminidase or key regulator of O-GlcNAc signaling?
Alonso J, Schimpl M, van Aalten DM. Alonso J, et al. J Biol Chem. 2014 Dec 12;289(50):34433-9. doi: 10.1074/jbc.R114.609198. Epub 2014 Oct 21. J Biol Chem. 2014. PMID: 25336650 Free PMC article. Review.
Cited by
- Dynamic O-GlcNAcylation and its roles in the cellular stress response and homeostasis.
Groves JA, Lee A, Yildirir G, Zachara NE. Groves JA, et al. Cell Stress Chaperones. 2013 Sep;18(5):535-58. doi: 10.1007/s12192-013-0426-y. Epub 2013 Apr 26. Cell Stress Chaperones. 2013. PMID: 23620203 Free PMC article. - Intracellular O-linked glycosylation directly regulates cardiomyocyte L-type Ca2+ channel activity and excitation-contraction coupling.
Ednie AR, Bennett ES. Ednie AR, et al. Basic Res Cardiol. 2020 Sep 10;115(6):59. doi: 10.1007/s00395-020-00820-0. Basic Res Cardiol. 2020. PMID: 32910282 - Modification of STIM1 by O-linked N-acetylglucosamine (O-GlcNAc) attenuates store-operated calcium entry in neonatal cardiomyocytes.
Zhu-Mauldin X, Marsh SA, Zou L, Marchase RB, Chatham JC. Zhu-Mauldin X, et al. J Biol Chem. 2012 Nov 9;287(46):39094-106. doi: 10.1074/jbc.M112.383778. Epub 2012 Sep 19. J Biol Chem. 2012. PMID: 22992728 Free PMC article. - Glucose deprivation-induced increase in protein O-GlcNAcylation in cardiomyocytes is calcium-dependent.
Zou L, Zhu-Mauldin X, Marchase RB, Paterson AJ, Liu J, Yang Q, Chatham JC. Zou L, et al. J Biol Chem. 2012 Oct 5;287(41):34419-31. doi: 10.1074/jbc.M112.393207. Epub 2012 Aug 20. J Biol Chem. 2012. PMID: 22908225 Free PMC article. - STIM1/Orai1-mediated SOCE: current perspectives and potential roles in cardiac function and pathology.
Collins HE, Zhu-Mauldin X, Marchase RB, Chatham JC. Collins HE, et al. Am J Physiol Heart Circ Physiol. 2013 Aug 15;305(4):H446-58. doi: 10.1152/ajpheart.00104.2013. Epub 2013 Jun 21. Am J Physiol Heart Circ Physiol. 2013. PMID: 23792674 Free PMC article. Review.
References
- Akimoto Y, Kawakami H, Yamamoto K, Munetomo E, Hida T, Hirano H. Elevated expression of O-GlcNAc-modified proteins and O-GlcNAc transferase in corneas of diabetic Goto-Kakizaki rats. Invest Ophthalmol Vis Sci 44: 3802–3809, 2003. - PubMed
- Bolli R, Becker L, Gross G, Mentzer R, Jr, Balshaw D, Lathrop DA. Myocardial protection at a crossroads: the need for translation into clinical therapy. Circ Res 95: 125–134, 2004. - PubMed
- Champattanachai V, Marchase RB, Chatham JC. Glucosamine protects neonatal cardiomyocytes from ischemia-reperfusion injury via increased protein-associated O-GlcNAc. Am J Physiol Cell Physiol 292: C178–C187, 2007. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials