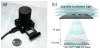Compact and light-weight automated semen analysis platform using lensfree on-chip microscopy - PubMed (original) (raw)
Compact and light-weight automated semen analysis platform using lensfree on-chip microscopy
Ting-Wei Su et al. Anal Chem. 2010.
Abstract
We demonstrate a compact and lightweight platform to conduct automated semen analysis using a lensfree on-chip microscope. This holographic on-chip imaging platform weighs ∼46 g, measures ∼4.2 × 4.2 × 5.8 cm, and does not require any lenses, lasers or other bulky optical components to achieve phase and amplitude imaging of sperms over ∼24 mm(2) field-of-view with an effective numerical aperture of ∼0.2. Using this wide-field lensfree on-chip microscope, semen samples are imaged for ∼10 s, capturing a total of ∼20 holographic frames. Digital subtraction of these consecutive lensfree frames, followed by appropriate processing of the reconstructed images, enables automated quantification of the count, the speed and the dynamic trajectories of motile sperms, while summation of the same frames permits counting of immotile sperms. Such a compact and lightweight automated semen analysis platform running on a wide-field lensfree on-chip microscope could be especially important for fertility clinics, personal male fertility tests, as well as for field use in veterinary medicine such as in stud farming and animal breeding applications.
Figures
Figure 1
(a) A lensfree holographic on-chip microscope that measures 4.2 × 4.2 × 5.8 cm and weighs 46 g is shown. The embedded light source (an LED filtered by a pinhole) and the CMOS image sensor are both powered through a USB connection from the side. This compact on-chip microscope can provide both amplitude and phase images of the sperms (loaded within a sliding tray) over a field-of-view of 24 mm2 with an effective numerical aperture of ~0.2. (b) A schematic diagram of the lensfree holographic microscope shown in (a) depicts the relative positions of the light source, the semen sample and the sensor chip. This schematic diagram is not drawn to scale.
Figure 2
(a) A digitally cropped lensfree hologram of an immobilized semen sample (6.05 million sperms per mL) that is acquired with the unit in Figure 1(a) is shown. (b) For comparison purposes, a bright-field microscope image of the same FOV as in (a) is acquired with a 40× objective-lens (NA = 0.65). (c) The amplitude image reconstructed from the raw hologram shown in (a) for the same FOV indicates the locations of the heads of the sperms. (d) The phase image reconstructed from the raw hologram shown in (a) for the same FOV illustrates both the heads and the tails of the sperms. (e) Automatic characterization results that are generated based on the reconstructed phase image in (d) are illustrated. The elliptical areas corresponding to sperm heads are enclosed by red circles while the tails are labeled with green lines. Defective sperms with missing or unusually curved tails (marked with the white arrows in (e)) are not reported toward positive sperm counts.
Figure 3
(a) A digitally subtracted lensfree hologram of three moving sperms is generated from two successive frames (500 ms apart) (b) A microscopic image, that is digitally reconstructed from the lensfree differential hologram shown in (a), illustrates the positions of three sperms in two successive frames (white spots show the sperms’ end positions and black spots show their starting positions). The displacement vectors of these sperms are labeled as S1, S2, and S3.
Figure 4
(a) Dynamic trajectories of 221 sperms within a field-of-view of ~24 mm2 are automatically tracked over a time-span of 10 s. The blue spots mark the end positions of the tracked sperms, while the green lines refer to their trajectories. (b) The speed histogram of these motile sperms is calculated using the information in (a) by summing the sperm displacements from all the consecutive frames and then dividing this sum by the total image acquisition duration.
Figure 5
Counting accuracy of the presented automated semen analysis platform for (a) immotile sperms; (b) motile sperms; and (c) both the motile and immotile sperms is illustrated at various sperm concentrations up to 12.5 × 106/mL. The _x_-axes are the sperm concentrations that are manually counted using a conventional bright-field microscope. The _y_-axes are the sperm concentrations that are automatically counted for the same semen samples using lensfree holographic images acquired with the on-chip microscope shown in Figure 1(a). The total counts in (c) are the summed up concentrations of the immotile sperms in (a) and the motile sperms in (b). Correlation coefficients (r) of these characterization results shown in (a), (b), and (c) (0.98, 0.99, and 0.98, respectively) further validate the accuracy of this compact and lightweight holographic lensfree microscope as a semen analysis platform.
Similar articles
- Holographic pixel super-resolution in portable lensless on-chip microscopy using a fiber-optic array.
Bishara W, Sikora U, Mudanyali O, Su TW, Yaglidere O, Luckhart S, Ozcan A. Bishara W, et al. Lab Chip. 2011 Apr 7;11(7):1276-9. doi: 10.1039/c0lc00684j. Epub 2011 Mar 1. Lab Chip. 2011. PMID: 21365087 Free PMC article. - Giga-pixel lensfree holographic microscopy and tomography using color image sensors.
Isikman SO, Greenbaum A, Luo W, Coskun AF, Ozcan A. Isikman SO, et al. PLoS One. 2012;7(9):e45044. doi: 10.1371/journal.pone.0045044. Epub 2012 Sep 12. PLoS One. 2012. PMID: 22984606 Free PMC article. - Lensfree microscopy on a cellphone.
Tseng D, Mudanyali O, Oztoprak C, Isikman SO, Sencan I, Yaglidere O, Ozcan A. Tseng D, et al. Lab Chip. 2010 Jul 21;10(14):1787-92. doi: 10.1039/c003477k. Epub 2010 May 6. Lab Chip. 2010. PMID: 20445943 Free PMC article. - Lensfree optofluidic microscopy and tomography.
Bishara W, Isikman SO, Ozcan A. Bishara W, et al. Ann Biomed Eng. 2012 Feb;40(2):251-62. doi: 10.1007/s10439-011-0385-3. Epub 2011 Sep 2. Ann Biomed Eng. 2012. PMID: 21887590 Review. - Partially coherent lensfree tomographic microscopy [Invited].
Isikman SO, Bishara W, Ozcan A. Isikman SO, et al. Appl Opt. 2011 Dec 1;50(34):H253-64. doi: 10.1364/AO.50.00H253. Appl Opt. 2011. PMID: 22193016 Free PMC article. Review.
Cited by
- Lens-free computational imaging of capillary morphogenesis within three-dimensional substrates.
Weidling J, Isikman SO, Greenbaum A, Ozcan A, Botvinick E. Weidling J, et al. J Biomed Opt. 2012 Dec;17(12):126018. doi: 10.1117/1.JBO.17.12.126018. J Biomed Opt. 2012. PMID: 23235893 Free PMC article. - Portable lensless wide-field microscopy imaging platform based on digital inline holography and multi-frame pixel super-resolution.
Sobieranski AC, Inci F, Tekin HC, Yuksekkaya M, Comunello E, Cobra D, von Wangenheim A, Demirci U. Sobieranski AC, et al. Light Sci Appl. 2015;4:e346. doi: 10.1038/lsa.2015.119. Epub 2015 Oct 23. Light Sci Appl. 2015. PMID: 29657866 Free PMC article. - Emerging technologies for home-based semen analysis.
Yu S, Rubin M, Geevarughese S, Pino JS, Rodriguez HF, Asghar W. Yu S, et al. Andrology. 2018 Jan;6(1):10-19. doi: 10.1111/andr.12441. Epub 2017 Dec 1. Andrology. 2018. PMID: 29194998 Free PMC article. Review. - On-chip cytometry using plasmonic nanoparticle enhanced lensfree holography.
Wei Q, McLeod E, Qi H, Wan Z, Sun R, Ozcan A. Wei Q, et al. Sci Rep. 2013;3:1699. doi: 10.1038/srep01699. Sci Rep. 2013. PMID: 23608952 Free PMC article. - Imaging without lenses: achievements and remaining challenges of wide-field on-chip microscopy.
Greenbaum A, Luo W, Su TW, Göröcs Z, Xue L, Isikman SO, Coskun AF, Mudanyali O, Ozcan A. Greenbaum A, et al. Nat Methods. 2012 Sep;9(9):889-95. doi: 10.1038/nmeth.2114. Epub 2012 Aug 30. Nat Methods. 2012. PMID: 22936170 Free PMC article.
References
- Björndahl L, Mortimer D, Barratt CLR, Castilla JA, Menkveld R, Kvist U, Alvarez JG, Haugen TB. A Practical Guide to Basic Laboratory Andrology. 1. Cambridge University Press; New York: 2010.
- Foote RH. J Anim Sci. 2002;80:1–10.
- World Health Organization (WHO) Laboratory Manual for the Examination of Human Semen and Sperm-Cervical Mucus Interaction. 4. Cambridge University Press; New York: 1999.
- Sokoloski JE, Blasco L, Storey BT, Wolf DP. Fertil Steril. 1977;28:1337–1341. - PubMed
- Atherton RW, Radany EW, Polakoski KL. Biol Reprod. 1978;18:624–628. - PubMed
Publication types
MeSH terms
Grants and funding
- R21 EB009222-02/EB/NIBIB NIH HHS/United States
- DP2 OD006427/OD/NIH HHS/United States
- 1R21EB009222-01/EB/NIBIB NIH HHS/United States
- R21 EB009222/EB/NIBIB NIH HHS/United States
- DP2 OD006427-01/OD/NIH HHS/United States
- R21 EB009222-01/EB/NIBIB NIH HHS/United States
- DP2OD006427/OD/NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources