Oseltamivir-resistant influenza A 2009 H1N1 virus in immunocompromised patients - PubMed (original) (raw)
Case Reports
Oseltamivir-resistant influenza A 2009 H1N1 virus in immunocompromised patients
Brianne A Couturier et al. Influenza Other Respir Viruses. 2010 Jul.
Abstract
Background: First-line treatment of influenza A 2009 H1N1 relies on neuraminidase inhibitors such as oseltamivir. Resistance conferred by the H275Y neuraminidase gene mutation is concerning and likely to increase.
Objectives: To characterize oseltamivir resistance in a hospital-based patient population.
Patients and methods: All available respiratory specimens positive for influenza A by direct fluorescent antibody, RT-PCR, or culture from patients at the University of Utah 5/09-12/09 were collected. Specimens were confirmed as 2009 H1N1 by the Utah Department of Health. RT-PCR and pyrosequencing were used to test for the H275Y mutation (CDC protocol). PyroMark Q24 AQ software (Qiagen, Valencia, CA, USA) was used to allow for quantitative H275Y mutation analysis. Medical records of patients with resistant virus were reviewed.
Results: We tested 191 influenza A virus-positive samples from 187 unique patients. Fifty (27%) patients were hospitalized. Four patient specimens (2.1%) were found to carry the H275Y mutation. Three patients were hospitalized, representing 6% of inpatient samples tested. Three patients had undergone hematopoietic stem cell transplant in the past year. Two patients died. Their influenza viruses were confirmed to be oseltamivir-resistant at an independent reference laboratory and through the Center for Disease Control and Prevention (CDC). One patient reported no history of prior oseltamivir exposure.
Conclusions: Widespread oseltamivir resistance among 2009 H1N1 remains a potential threat. Rapid techniques, such as pyrosequencing, which has the additional benefit of identifying mixed mutant populations of virus, may play a key role in identifying at-risk individuals and potentially unsuspected cases. Targeted surveillance of immunocompromised patients will be critical toward improving future influenza planning and therapy.
Figures
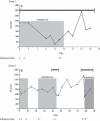
Figure 1
The course of antiviral treatment and absolute lymphocyte counts (ALC) over time are shown for Cases 1 and 2. Hospitalization is indicated by the black bar. (A) Case 1: Day 0 was the first positive test for influenza virus. Subsequent influenza virus tests are with (+). Resistant influenza virus (+*) was detected on day 9. The patient died on day 17. (B) Case 2: First positive influenza virus test was on day 0. Subsequent tests were performed on day 2, 9, 22, 42, 43, 77, and 78 and marked as (+, +*, or −). The sample from day 22 was submitted for resistance testing (+*), and results were reported on day 43 leading to discontinuation of oseltamivir therapy. The patient died on day 88.
Similar articles
- Detection of the rapid emergence of the H275Y mutation associated with oseltamivir resistance in severe pandemic influenza virus A/H1N1 09 infections.
Wang B, Dwyer DE, Blyth CC, Soedjono M, Shi H, Kesson A, Ratnamohan M, McPhie K, Cunningham AL, Saksena NK. Wang B, et al. Antiviral Res. 2010 Jul;87(1):16-21. doi: 10.1016/j.antiviral.2010.04.002. Epub 2010 Apr 10. Antiviral Res. 2010. PMID: 20385168 - Viral shedding and susceptibility to oseltamivir in hospitalized immunocompromised patients with influenza in the Influenza Resistance Information Study (IRIS).
Fraaij PL, Schutten M, Javouhey E, Burleigh L, Outlaw R, Kumar D, Boucher CA. Fraaij PL, et al. Antivir Ther. 2015;20(6):633-42. doi: 10.3851/IMP2957. Epub 2015 Apr 7. Antivir Ther. 2015. PMID: 25849228 Clinical Trial. - Molecular pathway of influenza pan-neuraminidase inhibitor resistance in an immunocompromised patient.
Abed Y, Schibler M, Checkmahomed L, Carbonneau J, Venable MC, Fage C, Giannotti F, Goncalves AR, Kaiser L, Boivin G. Abed Y, et al. Antivir Ther. 2019;24(8):581-587. doi: 10.3851/IMP3344. Antivir Ther. 2019. PMID: 32031540 - Emerging oseltamivir resistance in seasonal and pandemic influenza A/H1N1.
Renaud C, Kuypers J, Englund JA. Renaud C, et al. J Clin Virol. 2011 Oct;52(2):70-8. doi: 10.1016/j.jcv.2011.05.019. Epub 2011 Jun 17. J Clin Virol. 2011. PMID: 21684202 Review. - Emergence of oseltamivir resistance: control and management of influenza before, during and after the pandemic.
Dixit R, Khandaker G, Ilgoutz S, Rashid H, Booy R. Dixit R, et al. Infect Disord Drug Targets. 2013 Feb;13(1):34-45. doi: 10.2174/18715265112129990006. Infect Disord Drug Targets. 2013. PMID: 23675925 Review.
Cited by
- Respiratory viral infections in pediatric solid organ and hematopoietic stem cell transplantation.
Arslan D, Danziger-Isakov L. Arslan D, et al. Curr Infect Dis Rep. 2012 Dec;14(6):658-67. doi: 10.1007/s11908-012-0294-0. Curr Infect Dis Rep. 2012. PMID: 22968439 Free PMC article. - Viral Pneumonia in Patients with Hematopoietic Cell Transplantation and Hematologic Malignancies.
Green ML. Green ML. Clin Chest Med. 2017 Jun;38(2):295-305. doi: 10.1016/j.ccm.2016.12.009. Clin Chest Med. 2017. PMID: 28477640 Free PMC article. Review. - Approach to hematopoietic cell transplant candidates with respiratory viral detection.
Kim SR, Waghmare A, Hijano DR. Kim SR, et al. Front Pediatr. 2024 Jan 18;11:1339239. doi: 10.3389/fped.2023.1339239. eCollection 2023. Front Pediatr. 2024. PMID: 38304442 Free PMC article. Review. - Systematic review of influenza resistance to the neuraminidase inhibitors.
Thorlund K, Awad T, Boivin G, Thabane L. Thorlund K, et al. BMC Infect Dis. 2011 May 19;11:134. doi: 10.1186/1471-2334-11-134. BMC Infect Dis. 2011. PMID: 21592407 Free PMC article. - Reverse-transcription polymerase chain reaction/pyrosequencing to characterize neuraminidase H275 residue of influenza A 2009 H1N1 virus for rapid and specific detection of the viral oseltamivir resistance marker in a clinical laboratory.
Bao JR, Huard TK, Piscitelli AE, Tummala PR, Aleemi VE, Coon SL, Master RN, Lewinski MA, Clark RB. Bao JR, et al. Diagn Microbiol Infect Dis. 2011 Dec;71(4):396-402. doi: 10.1016/j.diagmicrobio.2011.09.003. Epub 2011 Oct 14. Diagn Microbiol Infect Dis. 2011. PMID: 22000086 Free PMC article.
References
- Centers for Disease Control and Prevention (CDC) . Patients hospitalized with 2009 pandemic influenza A (H1N1) – New York City, May. 2009. MMWR Morb Mortal Wkly Rep 2010; 58:1436–1440. - PubMed
- Uyeki T. Antiviral treatment for patients hospitalized with 2009 pandemic influenza A (H1N1). N Engl J Med 2009; 361:e110. - PubMed
- Dharan NJ, Gubareva LV, Meyer JJ et al. Infections with oseltamivir‐resistant influenza A(H1N1) virus in the United States. JAMA 2009; 301:1034–1041. - PubMed
- Hurt AC, Holien JK, Parker MW, Barr IG. Oseltamivir resistance and the H274Y neuraminidase mutation in seasonal, pandemic and highly pathogenic influenza viruses. Drugs 2009; 69:2523–2531. - PubMed
- Centers for Disease Control and Prevention (CDC) . Update: Influenza Activity – United States, August 30, 2009–January 9, 2010. MMWR Morb Mortal Wkly Rep 2010; 59:38–43. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous