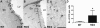Impaired adult olfactory bulb neurogenesis in the R6/2 mouse model of Huntington's disease - PubMed (original) (raw)
Impaired adult olfactory bulb neurogenesis in the R6/2 mouse model of Huntington's disease
Zacharias Kohl et al. BMC Neurosci. 2010.
Abstract
Background: Huntington's disease (HD) is an autosomal dominant neurodegenerative disorder linked to expanded CAG-triplet nucleotide repeats within the huntingtin gene. Intracellular huntingtin aggregates are present in neurons of distinct brain areas, among them regions of adult neurogenesis including the hippocampus and the subventricular zone/olfactory bulb system. Previously, reduced hippocampal neurogenesis has been detected in transgenic rodent models of HD. Therefore, we hypothesized that mutant huntingtin also affects newly generated neurons derived from the subventricular zone of adult R6/2 HD mice.
Results: We observed a redirection of immature neuroblasts towards the striatum, however failed to detect new mature neurons. We further analyzed adult neurogenesis in the granular cell layer and the glomerular layer of the olfactory bulb, the physiological target region of subventricular zone-derived neuroblasts. Using bromodeoxyuridine to label proliferating cells, we observed in both neurogenic regions of the olfactory bulb a reduction in newly generated neurons.
Conclusion: These findings suggest that the striatal environment, severely affected in R6/2 mice, is capable of attracting neuroblasts, however this region fails to provide sufficient signals for neuronal maturation. Moreover, in transgenic R6/2 animals, the hostile huntingtin-associated microenvironment in the olfactory bulb interferes with the survival and integration of new mature neurons. Taken together, endogenous cell repair strategies in HD may require additional factors for the differentiation and survival of newly generated neurons both in neurogenic and non-neurogenic regions.
Figures
Figure 1
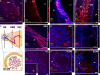
Expression pattern of mutant huntingtin in developing and mature neurons of the subventricular zone and the olfactory bulb in R6/2 mice. SVZ derived neurons originate from dividing GFAP expressing cells ("stem cells", B cells) that generate transit-amplifying cells (C cells) which in turn give rise to neuroblasts (A cells). DCX-expressing neuroblasts migrate along the RMS to the olfactory bulb and differentiate into different neuronal cell types in the GCL and the GLOM. Schematic representations of regions of adult neurogenesis in the SVZ/olfactory bulb (E, adapted from (35)). Aggregates of mutant huntingtin (green) are absent in GFAP expressing SVZ stem cells (red, A), EGF-R expressing transit-amplifying cells (red, B) and neuroblasts with DCX immunoreactivity (red, C), but appear in DARPP-32 positive mature neurons of the adjacent striatum (red, D) of R6/2 mice. Moreover, neuroblasts of the RMS (F), the GCL (G) and the GLOM (H) do not show immunoreactivity for mutant huntingtin. In contrast, aggregates of mutant huntingtin (green) were detected in mature, calretinin-expressing neurons (red) of the GCL (I) and the GLOM (J) and in TH expressing neurons of the GLOM (red, K) of the olfactory bulb. Topro-3 (blue) was used as counterstain for cell nuclei. Scale bar represents 20 μm.
Figure 2
R6/2 mice show increased migration of neuroblasts to the affected striatum compared to controls, without signs of substantial striatal neurogenesis. Representative images of DCX-expressing neuroblasts in wild-type (WT; A) and R6/2 mice (R6/2; B), higher magnification (from insert in B) of cells exhibiting heterogeneous morphologies (C): Some cells possess only short or no processes, some display multiple well-developed processes characteristic of migrating cells. Arrows indicate migrating DCX positive cells leaving the SVZ. Quantification of DCX-positive cells in the striatum of WT and R6/2 mice, predominantly in close proximity to the SVZ (D). Error bars represent SD, * indicates p < 0.05. Scale bars represent 50 μm.
Figure 3
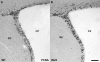
Cell proliferation in the subventricular zone of the R6/2 HD animals is unchanged compared to WT controls. Staining for dividing cells with the cell cycle marker PCNA revealed no difference in the amount of stem and precursor cell proliferation in the SVZ of WT (A) and R6/2 animals (B). Scale bar represents 20 μm.
Figure 4
Impaired survival of newly generated cells in the two neurogenic regions of the olfactory bulb in HD mice compared to controls. Significant reduction of BrdU-labeled newly generated cells in the GCL and the GLOM 4 weeks after BrdU administration in R6/2 mice compared to controls (C, G). Representative images of immunostainings for BrdU in the GCL (A, B) and the GLOM (E, F) of both groups. Schematic representation of the OB areas analyzed (D). Error bars represent SD, * indicates p < 0.05. Scale bars represent 50 μm.
Figure 5
The generation of new neurons in the neurogenic regions of the olfactory bulb is impaired in R6/2 animals compared to controls. In the GCL the percentage of newly generated cells that differentiate into a neuronal phenotype is unchanged comparing WT and R6/2 animals, as determined by double-labeling for BrdU and NeuN (A, B). Calculation of absolute numbers of surviving new neurons in the GCL revealed significantly decreased neurogenesis in HD mice compared to controls (C). Analysis of differentiation in the GLOM demonstrated unchanged neuronal differentiation in this region as well. Calculation of the total number of newly generated neurons in the GLOM showed a reduction of more than 50% in R6/2 mice compared to WT controls (F). D and E show representative immunostainings for BrdU and NeuN in the GLOM. Error bars represent SD, * indicates p < 0.05. Scale bars represent 20 μm.
Figure 6
Generation of dopaminergic neurons in the glomerular layer is less severely affected in HD mice compared to WT aminals. Further analysis of the phenotype of newly generated neurons in the GLOM of the olfactory bulb revealed that the proportion of new dopaminergic neurons (labeled for BrdU, NeuN and TH, A) was significantly increased in HD mice compared to controls (B). Scale bar represents 20 μm.
Figure 7
Increased cell death in the olfactory bulb of R6/2 mice. TUNEL staining of the GCL in the OB of WT and R6/2 animals shows an increase of TUNEL-positive profiles in transgenic animals carrying the mutant huntingtin (B) compared to WT mice (A). Scale bar represents 50 μm.
Similar articles
- Reduction in subventricular zone-derived olfactory bulb neurogenesis in a rat model of Huntington's disease is accompanied by striatal invasion of neuroblasts.
Kandasamy M, Rosskopf M, Wagner K, Klein B, Couillard-Despres S, Reitsamer HA, Stephan M, Nguyen HP, Riess O, Bogdahn U, Winkler J, von Hörsten S, Aigner L. Kandasamy M, et al. PLoS One. 2015 Feb 26;10(2):e0116069. doi: 10.1371/journal.pone.0116069. eCollection 2015. PLoS One. 2015. PMID: 25719447 Free PMC article. - Increased Olfactory Bulb BDNF Expression Does Not Rescue Deficits in Olfactory Neurogenesis in the Huntington's Disease R6/2 Mouse.
Smail S, Bahga D, McDole B, Guthrie K. Smail S, et al. Chem Senses. 2016 Mar;41(3):221-32. doi: 10.1093/chemse/bjv076. Epub 2016 Jan 18. Chem Senses. 2016. PMID: 26783111 Free PMC article. - Physical activity fails to rescue hippocampal neurogenesis deficits in the R6/2 mouse model of Huntington's disease.
Kohl Z, Kandasamy M, Winner B, Aigner R, Gross C, Couillard-Despres S, Bogdahn U, Aigner L, Winkler J. Kohl Z, et al. Brain Res. 2007 Jun 25;1155:24-33. doi: 10.1016/j.brainres.2007.04.039. Epub 2007 Apr 21. Brain Res. 2007. PMID: 17512917 - The selective vulnerability of nerve cells in Huntington's disease.
Sieradzan KA, Mann DM. Sieradzan KA, et al. Neuropathol Appl Neurobiol. 2001 Feb;27(1):1-21. doi: 10.1046/j.0305-1846.2001.00299.x. Neuropathol Appl Neurobiol. 2001. PMID: 11298997 Review. - Dynamic changes in the transcriptional profile of subventricular zone-derived postnatally born neuroblasts.
Khodosevich K, Alfonso J, Monyer H. Khodosevich K, et al. Mech Dev. 2013 Jun-Aug;130(6-8):424-32. doi: 10.1016/j.mod.2012.11.003. Epub 2012 Dec 5. Mech Dev. 2013. PMID: 23220001 Review.
Cited by
- Mutant Huntingtin promotes autonomous microglia activation via myeloid lineage-determining factors.
Crotti A, Benner C, Kerman BE, Gosselin D, Lagier-Tourenne C, Zuccato C, Cattaneo E, Gage FH, Cleveland DW, Glass CK. Crotti A, et al. Nat Neurosci. 2014 Apr;17(4):513-21. doi: 10.1038/nn.3668. Epub 2014 Mar 2. Nat Neurosci. 2014. PMID: 24584051 Free PMC article. - In Vivo Expression of Reprogramming Factor OCT4 Ameliorates Myelination Deficits and Induces Striatal Neuroprotection in Huntington's Disease.
Yu JH, Nam BG, Kim MG, Pyo S, Seo JH, Cho SR. Yu JH, et al. Genes (Basel). 2021 May 10;12(5):712. doi: 10.3390/genes12050712. Genes (Basel). 2021. PMID: 34068799 Free PMC article. - Ganglioside GD3 regulates neural stem cell quiescence and controls postnatal neurogenesis.
Fuchigami T, Itokazu Y, Yu RK. Fuchigami T, et al. Glia. 2024 Jan;72(1):167-183. doi: 10.1002/glia.24468. Epub 2023 Sep 5. Glia. 2024. PMID: 37667994 Free PMC article. - Neuronal replacement in the injured olfactory bulb.
Liu H, Guthrie KM. Liu H, et al. Exp Neurol. 2011 Apr;228(2):270-82. doi: 10.1016/j.expneurol.2011.01.021. Epub 2011 Feb 17. Exp Neurol. 2011. PMID: 21310147 Free PMC article. - Ganglioside GD3 regulates neural stem cell quiescence and controls postnatal neurogenesis.
Fuchigami T, Itokazu Y, Yu RK. Fuchigami T, et al. bioRxiv [Preprint]. 2023 Mar 14:2023.03.14.532547. doi: 10.1101/2023.03.14.532547. bioRxiv. 2023. PMID: 36993675 Free PMC article. Updated. Preprint.
References
- Mochel F, Charles P, Seguin F, Barritault J, Coussieu C, Perin L, Le Bouc Y, Gervais C, Carcelain G, Vassault A, Feingold J, Rabier D, Durr A. Early energy deficit in Huntington disease: identification of a plasma biomarker traceable during disease progression. PLoS One. 2007;2:e647. doi: 10.1371/journal.pone.0000647. - DOI - PMC - PubMed
- Paulsen JS, Langbehn DR, Stout JC, Aylward E, Ross CA, Nance M, Guttman M, Johnson S, MacDonald M, Beglinger LJ, Duff K, Kayson E, Biglan K, Shoulson I, Oakes D, Hayden M. Predict-HD Investigators and Coordinators of the Huntington Study Group. Detection of Huntington's disease decades before diagnosis: the Predict-HD study. JNNP. 2008;79:874–880. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical