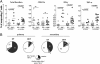Immunodominant T-cell responses to dengue virus NS3 are associated with DHF - PubMed (original) (raw)
Immunodominant T-cell responses to dengue virus NS3 are associated with DHF
Thaneeya Duangchinda et al. Proc Natl Acad Sci U S A. 2010.
Abstract
Dengue infections are increasing at an alarming rate in many tropical and subtropical countries, where epidemics can put health care systems under extreme pressure. The more severe infections lead to dengue hemorrhagic fever (DHF), which can be life threatening. A variety of viral and host factors have been associated with the severity of dengue infections. Because secondary dengue infection is more commonly associated with DHF than primary infections, the acquired immune response to dengue, both B cells and T cells have been implicated. In this study, we set out to study T-cell responses across the entire dengue virus proteome and to see whether these were related to disease severity in a cohort of dengue-infected children from Thailand. Robust responses were observed in most infected individuals against most viral proteins. Responses to NS3 were the most frequent, and there was a very strong association between the magnitude of the response and disease severity. Furthermore, in DHF, cytokine-high CD107a-negative cells predominated.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
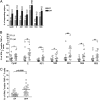
Fig. 1.
T-cell responses to the dengue proteome. Shown are representative plots for CD4+ and CD8+ T-cell subsets from PBMC stimulated with peptide pools covering the dengue proteome. (A) Gating strategy. Representative sets of responses for CD4 (B) and CD8 (C) T cells.
Fig. 2.
Breadth and magnitude of dengue specific T-cell responses. (A) T-cell responses against dengue virus were broad; the numbers above the bars represent the number of responding patients. (B) Magnitude of the responses to the peptide pools in the DF and DHF patient groups. (C) Sum of responses to all peptide pools for each patient. Differences between the two groups were tested by using the nonparametric two tailed Mann–Whitney test. Asterisks indicated P values: *P < 0.05; **P < 0.005; ***P < 0.0005.
Fig. 3.
NS3-specific responses in primary and secondary infections. (A) PBMC were stimulated with NS3 from the serotype of their current infection. Total responding cells (CD107a or IFN-γ or TNF-α) or individual responses to CD107a, IFN-γ, and TNF-α are shown. Differences between the two groups were tested by using the nonparametric two-tailed Mann–Whitney test. (B) Responding T cells from Fig. 3_A_ were divided into three groups: degranulation (CD107a) only, degranulation and cytokine production, and cytokine production only. Pie charts show the percentages of the functional cells to total responding T cells from patients in the DF and DHF groups. The average percentages were calculated by summing the percentages from individuals and dividing by number of cases.
Fig. 4.
MHC tetramer analysis of the HLA-A11-restricted NS3-GTS response. (A) PBMC from 10 secondary dengue-infected patients, 5 DF and 5 DHF (
Table S3
), were stained with the HLA-A*11-NS3-GTS tetramer corresponding to the serotype of their secondary infection. After stimulation with the same peptide used for tetramer staining and in the presence of anti-CD107a Ab, cells were stained for CD8, TNF-α, and IFN-γ. (B) Pie charts showing the details of the tetramer-specific responses by following the format of Fig. 3_B_.
Similar articles
- Association of dengue virus-specific polyfunctional T-cell responses with clinical disease severity in acute dengue infection.
Wijeratne DT, Fernando S, Gomes L, Jeewandara C, Jayarathna G, Perera Y, Wickramanayake S, Wijewickrama A, Ogg GS, Malavige GN. Wijeratne DT, et al. Immun Inflamm Dis. 2019 Dec;7(4):276-285. doi: 10.1002/iid3.271. Epub 2019 Sep 30. Immun Inflamm Dis. 2019. PMID: 31568656 Free PMC article. Clinical Trial. - Cross-reactive T-cell responses to the nonstructural regions of dengue viruses among dengue fever and dengue hemorrhagic fever patients in Malaysia.
Appanna R, Huat TL, See LL, Tan PL, Vadivelu J, Devi S. Appanna R, et al. Clin Vaccine Immunol. 2007 Aug;14(8):969-77. doi: 10.1128/CVI.00069-07. Epub 2007 Jun 13. Clin Vaccine Immunol. 2007. PMID: 17567768 Free PMC article. - Dominant recognition by human CD8+ cytotoxic T lymphocytes of dengue virus nonstructural proteins NS3 and NS1.2a.
Mathew A, Kurane I, Rothman AL, Zeng LL, Brinton MA, Ennis FA. Mathew A, et al. J Clin Invest. 1996 Oct 1;98(7):1684-91. doi: 10.1172/JCI118964. J Clin Invest. 1996. PMID: 8833919 Free PMC article. - T cell responses and dengue haemorrhagic fever.
Screaton G, Mongkolsapaya J. Screaton G, et al. Novartis Found Symp. 2006;277:164-71; discussion 171-6, 251-3. Novartis Found Symp. 2006. PMID: 17319161 Review. - Dengue hemorrhagic fever - A systemic literature review of current perspectives on pathogenesis, prevention and control.
Wang WH, Urbina AN, Chang MR, Assavalapsakul W, Lu PL, Chen YH, Wang SF. Wang WH, et al. J Microbiol Immunol Infect. 2020 Dec;53(6):963-978. doi: 10.1016/j.jmii.2020.03.007. Epub 2020 Mar 26. J Microbiol Immunol Infect. 2020. PMID: 32265181
Cited by
- Cross-serotypically conserved epitope recommendations for a universal T cell-based dengue vaccine.
Ahmed SF, Quadeer AA, Barton JP, McKay MR. Ahmed SF, et al. PLoS Negl Trop Dis. 2020 Sep 21;14(9):e0008676. doi: 10.1371/journal.pntd.0008676. eCollection 2020 Sep. PLoS Negl Trop Dis. 2020. PMID: 32956362 Free PMC article. - Longitudinal Analysis of Memory B and T Cell Responses to Dengue Virus in a 5-Year Prospective Cohort Study in Thailand.
Sánchez-Vargas LA, Kounlavouth S, Smith ML, Anderson KB, Srikiatkhachorn A, Ellison DW, Currier JR, Endy TP, Mathew A, Rothman AL. Sánchez-Vargas LA, et al. Front Immunol. 2019 Jun 13;10:1359. doi: 10.3389/fimmu.2019.01359. eCollection 2019. Front Immunol. 2019. PMID: 31263466 Free PMC article. - Antigenic cross-reactivity between Zika and dengue viruses: is it time to develop a universal vaccine?
Wen J, Shresta S. Wen J, et al. Curr Opin Immunol. 2019 Aug;59:1-8. doi: 10.1016/j.coi.2019.02.001. Epub 2019 Mar 15. Curr Opin Immunol. 2019. PMID: 30884384 Free PMC article. Review. - First experimental in vivo model of enhanced dengue disease severity through maternally acquired heterotypic dengue antibodies.
Ng JK, Zhang SL, Tan HC, Yan B, Martinez JM, Tan WY, Lam JH, Tan GK, Ooi EE, Alonso S. Ng JK, et al. PLoS Pathog. 2014 Apr 3;10(4):e1004031. doi: 10.1371/journal.ppat.1004031. eCollection 2014 Apr. PLoS Pathog. 2014. PMID: 24699622 Free PMC article. - Identification of Zika virus epitopes reveals immunodominant and protective roles for dengue virus cross-reactive CD8+ T cells.
Wen J, Tang WW, Sheets N, Ellison J, Sette A, Kim K, Shresta S. Wen J, et al. Nat Microbiol. 2017 Mar 13;2:17036. doi: 10.1038/nmicrobiol.2017.36. Nat Microbiol. 2017. PMID: 28288094 Free PMC article.
References
- World Health Organization . Dengue and Dengue Haemorrhagic Fever. Fact Sheet No. 117. Geneva: WHO; 2009.
- Halstead SB. Pathogenesis of dengue: Challenges to molecular biology. Science. 1988;239:476–481. - PubMed
- Nisalak A, et al. Serotype-specific dengue virus circulation and dengue disease in Bangkok, Thailand from 1973 to 1999. Am J Trop Med Hyg. 2003;68:191–202. - PubMed
- Suwandono A, et al. Four dengue virus serotypes found circulating during an outbreak of dengue fever and dengue haemorrhagic fever in Jakarta, Indonesia, during 2004. Trans R Soc Trop Med Hyg. 2006;100:855–862. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases