Drosophila IAP antagonists form multimeric complexes to promote cell death - PubMed (original) (raw)
Drosophila IAP antagonists form multimeric complexes to promote cell death
Cristinel Sandu et al. J Cell Biol. 2010.
Abstract
Apoptosis is a specific form of cell death that is important for normal development and tissue homeostasis. Caspases are critical executioners of apoptosis, and living cells prevent their inappropriate activation through inhibitor of apoptosis proteins (IAPs). In Drosophila, caspase activation depends on the IAP antagonists, Reaper (Rpr), Head involution defective (Hid), and Grim. These proteins share a common motif to bind Drosophila IAP1 (DIAP1) and have partially redundant functions. We now show that IAP antagonists physically interact with each other. Rpr is able to self-associate and also binds to Hid and Grim. We have defined the domain involved in self-association and demonstrate that it is critical for cell-killing activity in vivo. In addition, we show that Rpr requires Hid for recruitment to the mitochondrial membrane and for efficient induction of cell death in vivo. Both targeting of Rpr to mitochondria and forced dimerization strongly promotes apoptosis. Our results reveal the functional importance of a previously unrecognized multimeric IAP antagonist complex for the induction of apoptosis.
Figures
Figure 1.
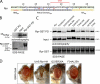
Rpr self-association and its impact on apoptotic activity. (A) Secondary structure consensus prediction of the Drosophila Rpr. Nomenclature: c, disordered; e, β-strand; h, helical. Three distinct Rpr domains are distinguishable: the IBM motif (IBMRpr), a central helical domain (Helical DomainRpr), and a C-terminal unstructured tail (TailRpr). The GH3 domain is marked with a red line above the amino acid sequence. Blue and red dots represent amino acids that were replaced by site-directed mutagenesis. Red dots represent amino acids that have an effect on the protein activities once replaced. (B) Pull-down (PD) experiment for testing the interaction between Rpr-GST and 35S-Rpr (Rpr-GST PD). As a specificity control, GST (bait) was tested for interaction with 35S-Rpr (GST PD). “Input” shows the expression of the radiolabeled Rpr and represents 10% of the protein amount used in the PD assay. “SDS-PAGE” indicates the amount of bait proteins used, as visualized by Coomassie staining. “Autoradiography” shows the radiolabeled proteins in the experiments. (C) Protein–protein interaction assay between Rpr-GST and 35S-Rpr mutants. “SDS-PAGE” indicates the amount of Rpr-GST protein used as bait. “Input” lanes indicate the autoradiography detection of the in vitro–translated 35S-Rpr mutants, for expression comparison. Each represents 10% of radiolabeled Rpr mutant amounts used in the PD assay. Rpr-GST PD is a pull-down assay, showing the binding of the individual 35S-Rpr mutants to Rpr-GST. (D) Eye images of transgenic Drosophila expressing Rpr-HA and Rpr-HA mutants Q23ER26A and the GH3 mutant F34AL35A. Genotypes: ;GMR>Gal4/+;, ;UAS:Rpr-HA/GMR>Gal4;, ;UAS:Rpr-HA Q23ER26A/GMR>Gal4;, ;UAS:Rpr-HA F34AL35A/GMR>Gal4;.
Figure 2.
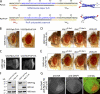
Enforced Rpr dimers kill by apoptosis in Drosophila. (A) Amino acid sequences and structural elements of Rpr dimers. RprLZ is an enforced parallel Rpr dimer where Rpr helical region (residues 10–46) was replaced with a parallel leucine zipper (GCN4), whereas RprProP is an enforced anti-parallel Rpr-dimer. LZ and ProP amino acid sequences are represented in blue. Residues in brown were inserted on both sides of each dimerization domain to preserve the same length as wild-type Rpr. IBMRpr and TailRpr are identical as in wild-type Rpr. A secondary structure prediction is represented below each sequence. Nomenclature: c, disordered; e, β-strand; h, helical. All constructs have attached a C-terminal HA tag, not represented in this diagram. To the right are schematic representations of RprLZ and RprProP with the IBMRpr (shown in red), ribbon representations of the dimerization domains LZ (PDB #2ZTA) and ProP (PDB #1R48) (shown in blue) and the TailRpr (shown in black). Note the position of the IBM motifs in RprLZ and RprProP. (B) Drosophila eye images from transgenic flies expressing RprLZ-HA or RprProP-HA. Genotypes: ;UAS:RprLZ-HA/GMR>Gal4; and ;UAS:RprProP-HA/GMR>Gal4;. (C) Eye-antennal imaginal discs from third instar transgenic larvae, expressing RprLZ-HA and RprProP-HA, stained with an anti-HA antibody. Genotype: UAS:p35/+;UAS:RprLZ-HA/GMR>Gal4; and UAS:p35/+;UAS:RprProP-HA/GMR>Gal4;. (D) Rescue of the RprLZ-HA induced eye ablation by Rpr-insensitive diap1 alleles or p35. Genotypes: ;UAS:RprLZ-HA/GMR>Gal4;, ;UAS:RprLZ-HA/GMR>Gal4;diap16-3s/+, ;UAS:RprLZ-HA/GMR>Gal4;diap123-4s/+ and UAS:p35/+;UAS:RprLZ-HA/GMR>Gal4;. (E) Rescue of the Rpr-HA induced eye ablation by Rpr-insensitive diap1 alleles or p35. Genotypes are identical to D, except that UAS:RprLZ-HA was replaced with UAS:Rpr-HA. (F) Ectopic expression of DIAP1ΔR-Flag or coexpression with Rpr-HA or RprLZ-HA in HEK293 cells, showing the ability of Rpr and RprLZ to induce DIAP1 degradation. Actin was used as a loading control. (G) Overexpression of RprLZ-HA in the presence of p35 in the posterior compartment of the wing discs and its effect on DIAP1 level. Expression of RprLZ was detected with an anti-HA antibody, whereas DIAP1 was immunostained with a rabbit anti-DIAP1 antibody.
Figure 3.
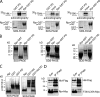
Rpr forms complexes with the other Drosophila IAP antagonists. (A) Pull-down assays for testing the interaction between Rpr-GST and 35S-Hid, 35S-Grim, or 35S-Skl (Rpr-GST PD). As specificity controls, pull-down experiments were performed between GST and 35S-Hid, 35S-Grim, or 35S-Skl (GST PD). “Input” lanes indicate the autoradiography detection of the in vitro–translated 35S-Hid, 35S-Grim, and 35S-Skl, for expression comparison. Each represents 10% of the radiolabeled protein amount used in the PD assay. “SDS-PAGE” shows the amount of GST or Rpr-GST used as bait. “Autoradiography” shows the phosphorimager detection of the radiolabeled proteins in the experiment. (B) Alternative pull-down experiments using purified components confirm Rpr self-association and show the interaction between Rpr and Hid lacking the mitochondrial targeting sequence. (Left) Specificity control experiment that shows the lack of interaction between Rpr-GST and ubiquitin (Rpr-GST PD). (Middle) Interaction between Rpr-GST and Rpr (Rpr-GST PD). (Right) Interaction between Rpr-GST and HidΔMTS (Rpr-GST PD). Rpr-GST (shown in the first lane of each panel) was incubated with purified ubiquitin, Rpr, or HidΔMTS (shown in the second lane of each panel). Protein complexes immobilized on glutathione Sepharose beads (Rpr-GST PD) are shown in the third lane of each panel. The proteins were separated by SDS-PAGE and visualized by Coomassie staining. (C) Reverse pull-down experiment showing the interaction between GST-Hid and Rpr. As a specificity control, GST failed to pull down Rpr. Purified Rpr protein (shown in the first lane of each panel) was incubated with either GST or GST-Hid (shown in the second lane of each panel). After incubation the complexes were pulled down using glutathione Sepharose beads (third lane of each panel). (D) Hid–Rpr and Hid–Rpr GH3 mutant F34AL35A coimmunoprecipitation experiment. Hid (anti-FLAG) does coimmunoprecipitate with Rpr (anti-Myc) from HEK293 cells (left) but not with the Rpr GH3 mutant F34AL35A (right). “Extr” represents the cell extract lane showing Hid-FLAG, Rpr-Myc, or GH3 mutant F34AL35A-Myc expression. “IP (FLAG)” represents the anti-FLAG immunoprecipitation fraction showing the level of Hid-FLAG, Rpr-Myc, or mutant levels in this fraction.
Figure 4.
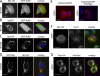
Rpr translocates to the mitochondria through physical interaction with Hid. (A) BT549 cells expressing Rpr-HA and GFP-XIAP. Rpr was stained with an anti-HA antibody. “Overlay” represents a composite image of Rpr (red), GFP-XIAP (green), and nuclei (DAPI, blue) staining. Bar, 20 µm. (B) BT549 cells transiently transfected with GFP-XIAP and mitochondrial RFP (mtRFP) plasmids. “Overlay” indicates GFP-XIAP (green), mitochondria (red), and nuclei (blue). Bar, 20 µm. (C) BT549 cells cotransfected with Hid-HA and GFP-XIAP plasmids. “Overlay” indicates Hid (red), GFP-XIAP (green), and nuclei (blue). Bar, 20 µm. (D) BT549 cells cotransfected with GFP-Rpr and Hid-HA plasmids. “Overlay” shows Rpr (green), Hid (red), and nuclei (blue) staining. Bar, 20 µm. (E) S2R+ Drosophila cells transiently transfected with a mCherryDIAP1 plasmid (left image) or with mCherryDIAP1 and Hid-Myc plasmids (right image). Each image shows the overlay of DIAP1 (red) and nuclei (blue) staining. Bar, 5 µm. (F) S2R+ Drosophila cell, transiently transfected with a Rpr-HA plasmid, followed by immunostaining with anti-HA and anti-Cyt C antibodies. “Overlay” indicates Rpr (red), Cyt C (green), and nuclei (blue) staining. Bar, 5 µm. (G) S2R+ Drosophila cell cotransfected with Rpr-HA and Hid-Myc plasmids. Cells were immunostained with an anti-HA antibody and an anti-Myc antibody. “Overlay” represents Rpr (green), Hid (red), and nuclei (blue) staining. Bar, 5 µm.
Figure 5.
Hid and Rpr act cooperatively to induce cell death in Drosophila. (A) Rescue of Rpr-induced eye ablation by Hid RNAi and Rpr RNAi. A rough Drosophila eye caused by overexpression of Rpr (left) is suppressed when RNAi transgenes knock down either Hid (UAS-CG5123 RNAi; middle) or Rpr (UAS-CG4319 RNAi; right). Genotypes: (left) ;GMR>Gal4/+;GMR>Rpr/+, (middle) ;GMR>Gal4/+;UAS:Hid RNAi/GMR>Rpr, (right) ;GMR>Gal4/+;UAS-Rpr RNAi/GMR>Rpr. (B) Hid-induced eye ablation (top image) is suppressed through UAS-Hid RNAi (bottom image). Genotypes: (top) GMR>Gal4/+;GMR-Hid/+;, (bottom) GMR>Gal4/+;GMR>Hid/+;UAS:Hid RNAi/+. (C) Anti-HA immunolabeling of Drosophila S2R+ cells transiently transfected with a Rpr-HA plasmid alone (left), or together with 200 nM siRNA directed against Hid mRNA (right). Bar, 100 µm. (D) Quantification of Rpr-HA–positive cells, in the absence or presence of Hid siRNA in S2R+ transient transfection experiments (top). Percentages of Rpr-positive cells were calculated by counting of at least 1,000 cells for each sample. Efficiency of Hid siRNA as assessed through anti-Myc Western blot of ectopically expressed Hid-Myc in the presence of Hid siRNA in S2R+ cells. (E) Rescue of the Rpr-induced eye ablation by the hidA206 and hidWR+X1 alleles. (First two images) Comparison of Rpr eye phenotype without or with the hidA206 allele. Genotypes: ;GMR>Gal4/+;GMR>Rpr/+ and ;GMR>Gal4/+;GMR>Rpr/hidA206. (Last two images) Rpr eye phenotype without or with hidWR+X1 allele. Genotypes: ;GMR>Gal4/+;GMR>Rpr/+ and ;GMR>Gal4/+;GMR>Rpr/hidW+RX1.
Figure 6.
DIAP1 auto-ubiquitination and interaction with Rpr and Hid. (A) SDS-PAGE gel showing E1 ubiquitin-activating enzyme Uba1 (Uba1-GST), E2 ubiquitin-conjugating enzyme UbcD1 (6His-UbcD1), 6His-ubiquitin (Ub), E3 ubiquitin ligase DIAP1 (6His-Flag-DIAP1), Rpr-His6, and HidΔMTS-His6, used in ubiquitination assays. Purification tags are not shown in the figure labeling. (B) In vitro coupling of Ub on UbcD1 (E2) in the absence (lane 1) or presence of Mg2+-ATP (lane 2). UbcD1-Ub adduct was detected by Coomassie staining. (C) In vitro DIAP1 auto-ubiquitination. Ubiquitination reactions containing E1, E2, Ub, and Flag-DIAP1, in the absence of Mg2+-ATP (lane 1) or in the presence of Mg2+-ATP (lane 2). The reaction was supplemented additionally with Rpr (lane 3), HidΔMTS (lane 4), or both (lane 5). Flag-DIAP1 was immunoprecipitated with anti-FLAG resin. Polyubiquitination species were detected in Western blot with an anti-ubiquitin antibody. (D) Coomassie-stained SDS-PAGE gel, showing the coimmunoprecipitation of Flag-DIAP1 with Rpr and HidΔMTS. “Input” shows the amount of Flag-DIAP1 (lane 1), Rpr (lane 2), or HidΔMTS (lane 3) used for co-immunoprecipitation. “IP:Flag” shows the anti-FLAG coimmunoprecipitation fractions. Lane 4 indicates the amount of Flag-DIAP1 recovered by the anti-FLAG resin. Lane 5 shows the coimmunoprecipitation of Rpr with Flag-DIAP1. Lane 6 shows the coimmunoprecipitation of HidΔMTS with Flag-DIAP1. Lane 7 shows the coimmunoprecipitation of HidΔMTS and Rpr with Flag-DIAP1.
Figure 7.
Mitochondrial targeting enhances Rpr’s killing capacity and its stability. (A) Expression of transgenic Rpr-MTS or GH3 mutant F34L35-MTS in Drosophila eyes induces severe eye ablation. Rpr-MTS and F34AL35A-MTS constructs consist of full-length Rpr or mutant (residues 1–65), followed by an HA tag and the Hid MTS (residues 387–410). Genotypes: ;UAS:Rpr-MTS/GMR>Gal4; and ;UAS:Rpr-MTS F34AL35A/GMR>Gal4;. (B) Third instar eye-antennal discs, stained with an anti-active caspase antibody. mf line indicates the position of the morphogenetic furrow. Genotypes: ;UAS:Rpr-MTS/GMR>Gal4; and ;UAS:Rpr-MTS F34AL35A/GMR>Gal4;. (C) Rpr-MTS and F34L35-MTS induce DIAP1ΔR degradation in HEK293 cells. DIAP1ΔR was expressed alone or coexpressed with Rpr-MTS or F34L35-MTS in HEK293 cells. The level or DIAP1ΔR, Rpr, and F34L35 mutant were assessed by Western blotting. Actin was used as a loading control. (D) Hid has a stabilizing role on Rpr’s protein level in HEK293 cells. (Lane 1) Rpr protein level in HEK293 cell extracts, after transient cotransfection of a Rpr-HA and YFP-Mem constructs. (Lane 2) Hid protein level in cell extracts of HEK293 after transient cotransfection of a Hid-Flag and YFP-Mem constructs. (Lane 3) Rpr and Hid protein levels in HEK293 cell extract, after transient cotransfection with Rpr-HA, Hid-Flag, and YFP-Mem plasmids. YFP-Mem was used as a transfection control. (E) Representation of Rpr and Hid cooperative induction of cell death. Left diagram, Rpr homomers interact with DIAP1 and induce its ubiquitination in the cytoplasm. Conversely, DIAP1 induces Rpr ubiquitination. Cell death or protection from death is dependent on the balance between Rpr and DIAP1. Other factors (O.F.) such as Grim, Skl, or others could be part of this complex. (Right) Rpr forms a complex with Hid at the mitochondrial membrane. In this complex Rpr accumulates, and leads to a more efficient DIAP1 degradation, which tips the balance toward cell death. Other factors (O.F.) such as Grim or members of the Bcl2 family proteins (Debcl and Buffy) could be interacting in this mitochondrial complex.
Similar articles
- Mitochondrial localization of Reaper to promote inhibitors of apoptosis protein degradation conferred by GH3 domain-lipid interactions.
Freel CD, Richardson DA, Thomenius MJ, Gan EC, Horn SR, Olson MR, Kornbluth S. Freel CD, et al. J Biol Chem. 2008 Jan 4;283(1):367-379. doi: 10.1074/jbc.M708931200. Epub 2007 Nov 12. J Biol Chem. 2008. PMID: 17998202 - The Drosophila inhibitor of apoptosis (IAP) DIAP2 is dispensable for cell survival, required for the innate immune response to gram-negative bacterial infection, and can be negatively regulated by the reaper/hid/grim family of IAP-binding apoptosis inducers.
Huh JR, Foe I, Muro I, Chen CH, Seol JH, Yoo SJ, Guo M, Park JM, Hay BA. Huh JR, et al. J Biol Chem. 2007 Jan 19;282(3):2056-68. doi: 10.1074/jbc.M608051200. Epub 2006 Oct 26. J Biol Chem. 2007. PMID: 17068333 - Hid, Rpr and Grim negatively regulate DIAP1 levels through distinct mechanisms.
Yoo SJ, Huh JR, Muro I, Yu H, Wang L, Wang SL, Feldman RM, Clem RJ, Müller HA, Hay BA. Yoo SJ, et al. Nat Cell Biol. 2002 Jun;4(6):416-24. doi: 10.1038/ncb793. Nat Cell Biol. 2002. PMID: 12021767 - Cell death regulation by the mammalian IAP antagonist Diablo/Smac.
Verhagen AM, Vaux DL. Verhagen AM, et al. Apoptosis. 2002 Apr;7(2):163-6. doi: 10.1023/a:1014318615955. Apoptosis. 2002. PMID: 11865200 Review. - Regulation of apoptosis in Drosophila.
Steller H. Steller H. Cell Death Differ. 2008 Jul;15(7):1132-8. doi: 10.1038/cdd.2008.50. Epub 2008 Apr 25. Cell Death Differ. 2008. PMID: 18437164 Review.
Cited by
- Mitochondria in apoptosis: Bcl-2 family members and mitochondrial dynamics.
Martinou JC, Youle RJ. Martinou JC, et al. Dev Cell. 2011 Jul 19;21(1):92-101. doi: 10.1016/j.devcel.2011.06.017. Dev Cell. 2011. PMID: 21763611 Free PMC article. Review. - CDK7 regulates the mitochondrial localization of a tail-anchored proapoptotic protein, Hid.
Morishita J, Kang MJ, Fidelin K, Ryoo HD. Morishita J, et al. Cell Rep. 2013 Dec 26;5(6):1481-8. doi: 10.1016/j.celrep.2013.11.030. Epub 2013 Dec 19. Cell Rep. 2013. PMID: 24360962 Free PMC article. - Engorgement of Rhipicephalus haemaphysaloides ticks blocked by silencing a protein inhibitor of apoptosis.
Tuerdi M, Hu S, Wang Y, Zhou Y, Cao J, Zhang H, Zhou J. Tuerdi M, et al. Exp Appl Acarol. 2021 Jul;84(3):623-636. doi: 10.1007/s10493-021-00637-z. Epub 2021 Jun 16. Exp Appl Acarol. 2021. PMID: 34136982 - Drosophila BRUCE inhibits apoptosis through non-lysine ubiquitination of the IAP-antagonist REAPER.
Domingues C, Ryoo HD. Domingues C, et al. Cell Death Differ. 2012 Mar;19(3):470-7. doi: 10.1038/cdd.2011.116. Epub 2011 Sep 2. Cell Death Differ. 2012. PMID: 21886178 Free PMC article. - Targeting Drosophila Sas6 to mitochondria reveals its high affinity for Gorab.
Kovacs L, Fatalska A, Glover DM. Kovacs L, et al. Biol Open. 2022 Nov 1;11(11):bio059545. doi: 10.1242/bio.059545. Epub 2022 Nov 18. Biol Open. 2022. PMID: 36331102 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials