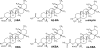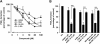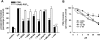Inhibition of microsomal prostaglandin E2 synthase-1 as a molecular basis for the anti-inflammatory actions of boswellic acids from frankincense - PubMed (original) (raw)
. 2011 Jan;162(1):147-62.
doi: 10.1111/j.1476-5381.2010.01020.x.
A Koeberle, A Rossi, F Dehm, M Verhoff, S Reckel, T J Maier, J Jauch, H Northoff, F Bernhard, V Doetsch, L Sautebin, O Werz
Affiliations
- PMID: 20840544
- PMCID: PMC3012413
- DOI: 10.1111/j.1476-5381.2010.01020.x
Inhibition of microsomal prostaglandin E2 synthase-1 as a molecular basis for the anti-inflammatory actions of boswellic acids from frankincense
U Siemoneit et al. Br J Pharmacol. 2011 Jan.
Abstract
Background and purpose: Frankincense, the gum resin derived from Boswellia species, showed anti-inflammatory efficacy in animal models and in pilot clinical studies. Boswellic acids (BAs) are assumed to be responsible for these effects but their anti-inflammatory efficacy in vivo and their molecular modes of action are incompletely understood.
Experimental approach: A protein fishing approach using immobilized BA and surface plasmon resonance (SPR) spectroscopy were used to reveal microsomal prostaglandin E(2) synthase-1 (mPGES1) as a BA-interacting protein. Cell-free and cell-based assays were applied to confirm the functional interference of BAs with mPGES1. Carrageenan-induced mouse paw oedema and rat pleurisy models were utilized to demonstrate the efficacy of defined BAs in vivo.
Key results: Human mPGES1 from A549 cells or in vitro-translated human enzyme selectively bound to BA affinity matrices and SPR spectroscopy confirmed these interactions. BAs reversibly suppressed the transformation of prostaglandin (PG)H(2) to PGE(2) mediated by mPGES1 (IC(50) = 3-10 µM). Also, in intact A549 cells, BAs selectively inhibited PGE(2) generation and, in human whole blood, β-BA reduced lipopolysaccharide-induced PGE(2) biosynthesis without affecting formation of the COX-derived metabolites 6-keto PGF(1α) and thromboxane B(2) . Intraperitoneal or oral administration of β-BA (1 mg·kg(-1) ) suppressed rat pleurisy, accompanied by impaired levels of PGE(2) and β-BA (1 mg·kg(-1) , given i.p.) also reduced mouse paw oedema, both induced by carrageenan.
Conclusions and implications: Suppression of PGE(2) formation by BAs via interference with mPGES1 contribute to the anti-inflammatory effectiveness of BAs and of frankincense, and may constitute a biochemical basis for their anti-inflammatory properties.
© 2010 The Authors. British Journal of Pharmacology © 2010 The British Pharmacological Society.
Figures
Figure 1
Chemical structures of boswellic acids (BAs) and α-amyrin. 3-_O_-Acetyl-β-boswellic acid (Aβ-BA); 3-_O_-acetyl-11-keto-β-boswellic acid (AKBA); β-boswellic acid (β-BA); 11-keto-β-boswellic acid (KBA); 3-_O_-oxaloyl-11-β-keto-boswellic acid (ox-KBA).
Figure 2
Boswellic acids (BAs) bind to microsomal prostaglandin E2 synthase 1 (mPGES1). (A) Supernatants of A549 cell lysates were incubated with β-BA-Seph, KBA-Seph or with Seph, as indicated. (B) Purified, _in vitro_-translated mPGES1 (200 ng) was incubated with β-BA-Seph, KBA-Seph or Seph beads. Precipitated proteins were separated by SDS-PAGE, and visualized by Western blotting, using specific antibodies against mPGES1 (A,B) or COX-2 (A). An aliquot of the supernatant was used as positive control. Similar results were obtained in three additional experiments. KBA, 11-keto-β-boswellic acid; SDS-PAGE, sodium dodecyl sulphate polyacrylamide gel electrophoresis.
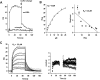
Figure 3
Analysis of the binding of boswellic acids to microsomal prostaglandin E2 synthase 1 (mPGES1) by surface plasmon resonance spectroscopy. _In vitro_-translated mPGES1 was coupled to a CM5 biosensor chip and 3-_O_-oxaloyl-KBA was used as analyte. Specific binding profiles were obtained after subtracting the signal [response units (RU)] from the untreated control cell. (A) Binding of 3-_O_-oxaloyl-KBA (ox-KBA) and α-amyrin (10 µM each) to mPGES1. (B) Binding curves for 3-_O_-oxaloyl-KBA. The equilibrium responses (Req) for 3-_O_-oxaloyl-KBA at different concentrations were plotted versus the concentration of the compound. (C) Kinetic analysis of 3-_O_-oxaloyl-KBA-binding to mPGES1. Representative sensograms for the injection of 0.5 µM up to 25 µM 3-_O_-oxaloyl-KBA are shown. A general analysis was applied to fit the data to a 1:1 binding model (bold lines), and the quality of the fit is displayed by the plots of the residuals. Results are representative for at least three independent experiments. KBA, 11-keto-β-boswellic acid.
Figure 4
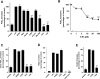
Effects of boswellic acids (BAs) on the activity of microsomal prostaglandin E2 synthase 1 (mPGES1) in a cell-free assay. (A) Concentration-response analysis. Microsomal preparations of IL-1β-stimulated A549 cells were pre-incubated with vehicle (DMSO) or BAs for 15 min at 4°C. PGH2 was added and after 1 min, the reaction was stopped and PGE2 was analysed by RP-HPLC as described. The 100% value corresponds to 944 ± 118 pmol PGE2 formed. (B) Reversibility of mPGES1 inhibition. Microsomal fractions of IL-1β-stimulated A549 cells were pre-incubated with 3 µM MK-886 or 10 µM BAs. An aliquot was diluted 10-fold to obtain an inhibitor concentration of 0.3 and 1 µM respectively. For comparison, microsomal preparations were pre-incubated with 0.3 µM MK-886, 1 µM BA or with vehicle (veh, DMSO), and then, 20 µM PGH2 was added (no dilution). After 1 min, PGE2 formation was analysed by RP-HPLC. Data are given as mean + SE, n = 3–4, ***P < 0.001 versus vehicle (DMSO) control. DMSO, dimethyl sulphoxide; RP-HPLC, reversed phase-high performance liquid chromatography.
Figure 5
Effects of boswellic acids (BAs) on PGE2 and 6-keto PGF1α formation in intact A549 cells. IL-1β-treated A549 cells were pre-incubated (A) with vehicle (veh, DMSO), BAs (30 µM each), α-amyrin (30 µM), MK-886 (30 µM) or celecoxib (cele, 5 µM) (B) with BAs at the indicated concentrations. After 10 min at 37°C, 2.5 µM A23187 plus 1 µM AA and [3H]AA (18.4 kBq) were added (or left untreated = unstim.) and after another 15 min, formed [3H]PGE2 was analysed as described in the Methods. 6-Keto PGF1α was analysed using High Sensitivity EIA Kits; the 100% value corresponds to 87 ± 11 pg 106 cells. Data are given as mean + SE, n = 3–5. ***P < 0.001 versus vehicle (DMSO) control. AA; arachidonic acid; DMSO, dimethyl sulphoxide.
Figure 6
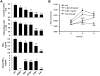
Effects of boswellic acids (BAs) on prostanoid biosynthesis in human whole blood. Heparinized human whole blood, treated with 1 µM CV4152 and 50 µM aspirin, was pre-incubated with (A) BAs and α-amyrin (10 µM, each) or vehicle (veh, DMSO) for 10 min at RT and then 10 µg·mL−1 LPS was added (or left untreated = unstim.). After 5 h at 37°C, PGE2 was separated by RP-HPLC and quantified by EIA. Controls: MK-886 (30 µM), indomethacin (indo, 50 µM), and celecoxib (cele, 20 µM). (B) Concentration-response of β-BA. (C,D) 6-keto PGF1α and TXB2 formation. 6-Keto PGF1α (C) was directly determined in blood plasma from samples above (see A) incubated with β-BA or AKBA (10 µM, each), indo (50 µM), cele (20 µM) or veh (DMSO). Inhibition of TXB2 formation (D) was assessed in heparinized human whole blood without CV4152 and aspirin. Both 6-keto PGF1α and TXB2 were measured by EIA. The 100% values corresponds to 221.8 ± 19.7 pg·mL−1 PGE2, 382.5 ± 22.3 pg·mL−1 6-keto PGF1α, and 37.9 ± 4.4 ng·mL−1 TXB2 respectively. (E) 12-HHT formation. Heparinized human blood was pre-incubated with β-BA (50 µM), indo (20 µM) or veh (DMSO) for 10 min, and A23187 (30 µM) was added (or left untreated = unstim.). After 10 min at 37°C, 12-HHT was analysed by HPLC. The 100% value corresponds to 148.8 ± 16.7 ng·mL−1 12-HHT. Data are given as mean + SE, n = 4–5; *P < 0.05; ***P < 0.001 versus vehicle (0.1% DMSO) control. β-BA, β-boswellic acid; AKBA, 3-O-acetyl-11-keto-β-boswellic acid; DMSO, dimethyl sulphoxide; RP-HPLC, reversed phase-high performance liquid chromatography.
Figure 7
Effects of boswellic acids (BAs) in animal models in vivo. (A) Carrageenan-induced pleurisy in rats. Thirty minutes before intrapleural injection of carrageenan, rats (n = 10 for each experimental group) were treated i.p. with BAs (1 mg·kg−1 each), indomethacin (5 mg·kg−1) or vehicle (veh, DMSO 4%). Exudate volume, PGE2 and 6-keto PGF1α levels as well as inflammatory cell accumulation in pleural cavity were assessed 4 h after carrageenan injection. Data are expressed as mean ± SE, n = 10. *P < 0.05; **P < 0.01; ***P < 0.01 versus vehicle; n.d. = not determined. (B) Carrageenan-induced mouse paw oedema. Animals (n = 10 for each experimental group) were treated i.p. with 0.25 and 1 mg·kg−1β-BA, 5 mg·kg−1 indomethacin (indo) or veh (2% DMSO) 30 min before carrageenan subplantar injection. Data are given as mean + SE, *P < 0.05; **P < 0.01; ***P < 0.001 versus vehicle control. DMSO, dimethyl sulphoxide.
Similar articles
- Boswellia serrata: an overall assessment of in vitro, preclinical, pharmacokinetic and clinical data.
Abdel-Tawab M, Werz O, Schubert-Zsilavecz M. Abdel-Tawab M, et al. Clin Pharmacokinet. 2011 Jun;50(6):349-69. doi: 10.2165/11586800-000000000-00000. Clin Pharmacokinet. 2011. PMID: 21553931 Review. - Tetra- and pentacyclic triterpene acids from the ancient anti-inflammatory remedy frankincense as inhibitors of microsomal prostaglandin E(2) synthase-1.
Verhoff M, Seitz S, Paul M, Noha SM, Jauch J, Schuster D, Werz O. Verhoff M, et al. J Nat Prod. 2014 Jun 27;77(6):1445-51. doi: 10.1021/np500198g. Epub 2014 May 20. J Nat Prod. 2014. PMID: 24844534 Free PMC article. - The molecular pharmacology and in vivo activity of 2-(4-chloro-6-(2,3-dimethylphenylamino)pyrimidin-2-ylthio)octanoic acid (YS121), a dual inhibitor of microsomal prostaglandin E2 synthase-1 and 5-lipoxygenase.
Koeberle A, Rossi A, Zettl H, Pergola C, Dehm F, Bauer J, Greiner C, Reckel S, Hoernig C, Northoff H, Bernhard F, Dötsch V, Sautebin L, Schubert-Zsilavecz M, Werz O. Koeberle A, et al. J Pharmacol Exp Ther. 2010 Mar;332(3):840-8. doi: 10.1124/jpet.109.160663. Epub 2009 Nov 24. J Pharmacol Exp Ther. 2010. PMID: 19934399 - Boswellic acids from frankincense inhibit lipopolysaccharide functionality through direct molecular interference.
Henkel A, Kather N, Mönch B, Northoff H, Jauch J, Werz O. Henkel A, et al. Biochem Pharmacol. 2012 Jan 1;83(1):115-21. doi: 10.1016/j.bcp.2011.09.026. Epub 2011 Oct 5. Biochem Pharmacol. 2012. PMID: 22001311 - Modulation of the immune system by Boswellia serrata extracts and boswellic acids.
Ammon HP. Ammon HP. Phytomedicine. 2010 Sep;17(11):862-7. doi: 10.1016/j.phymed.2010.03.003. Epub 2010 Aug 8. Phytomedicine. 2010. PMID: 20696559 Review.
Cited by
- The Beneficial Effect of Boswellic Acid on Bone Metabolism and Possible Mechanisms of Action in Experimental Osteoporosis.
Al-Dhubiab BE, Patel SS, Morsy MA, Duvva H, Nair AB, Deb PK, Shah J. Al-Dhubiab BE, et al. Nutrients. 2020 Oct 18;12(10):3186. doi: 10.3390/nu12103186. Nutrients. 2020. PMID: 33081068 Free PMC article. - Pharmacological inhibition of microsomal prostaglandin E synthase-1 suppresses epidermal growth factor receptor-mediated tumor growth and angiogenesis.
Finetti F, Terzuoli E, Bocci E, Coletta I, Polenzani L, Mangano G, Alisi MA, Cazzolla N, Giachetti A, Ziche M, Donnini S. Finetti F, et al. PLoS One. 2012;7(7):e40576. doi: 10.1371/journal.pone.0040576. Epub 2012 Jul 18. PLoS One. 2012. PMID: 22815767 Free PMC article. - Boswellia serrata: an overall assessment of in vitro, preclinical, pharmacokinetic and clinical data.
Abdel-Tawab M, Werz O, Schubert-Zsilavecz M. Abdel-Tawab M, et al. Clin Pharmacokinet. 2011 Jun;50(6):349-69. doi: 10.2165/11586800-000000000-00000. Clin Pharmacokinet. 2011. PMID: 21553931 Review. - A boswellic acid-containing extract attenuates hepatic granuloma in C57BL/6 mice infected with Schistosoma japonicum.
Liu M, Chen P, Büchele B, Dong S, Huang D, Ren C, Zhang Y, Hou X, Simmet T, Shen J. Liu M, et al. Parasitol Res. 2013 Mar;112(3):1105-11. doi: 10.1007/s00436-012-3237-7. Epub 2012 Dec 28. Parasitol Res. 2013. PMID: 23271565 - Effects of Frankincense Compounds on Infection, Inflammation, and Oral Health.
Almeida-da-Silva CLC, Sivakumar N, Asadi H, Chang-Chien A, Qoronfleh MW, Ojcius DM, Essa MM. Almeida-da-Silva CLC, et al. Molecules. 2022 Jun 29;27(13):4174. doi: 10.3390/molecules27134174. Molecules. 2022. PMID: 35807419 Free PMC article. Review.
References
- Ammon HP. Boswellic acids in chronic inflammatory diseases. Planta Med. 2006;72:1100–1116. - PubMed
- Asano K, Lilly CM, Drazen JM. Prostaglandin G/H synthase-2 is the constitutive and dominant isoform in cultured human lung epithelial cells. Am J Physiol. 1996;271:L126–L131. - PubMed
- Buchele B, Simmet T. Analysis of 12 different pentacyclic triterpenic acids from frankincense in human plasma by high-performance liquid chromatography and photodiode array detection. J Chromatogr B Analyt Technol Biomed Life Sci. 2003;795:355–362. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources