Adiponectin protects rat hippocampal neurons against excitotoxicity - PubMed (original) (raw)
doi: 10.1007/s11357-010-9173-5. Epub 2010 Sep 15.
Ruiqian Wan, Jingping Hu, Mark P Mattson, Edward Spangler, Shan Liu, Suk-Yu Yau, Tatia M C Lee, Marc Gleichmann, Donald K Ingram, Kwok-Fai So, Sige Zou
Affiliations
- PMID: 20842535
- PMCID: PMC3127462
- DOI: 10.1007/s11357-010-9173-5
Adiponectin protects rat hippocampal neurons against excitotoxicity
Guang Qiu et al. Age (Dordr). 2011 Jun.
Abstract
Adiponectin exerts multiple regulatory functions in the body and in the hypothalamus primarily through activation of its two receptors, adiponectin receptor1 and adiponectin receptor 2. Recent studies have shown that adiponectin receptors are widely expressed in other areas of the brain including the hippocampus. However, the functions of adiponectin in brain regions other than the hypothalamus are not clear. Here, we report that adiponectin can protect cultured hippocampal neurons against kainic acid-induced (KA) cytotoxicity. Adiponectin reduced the level of reactive oxygen species, attenuated apoptotic cell death, and also suppressed activation of caspase-3 induced by KA. Pretreatment of hippocampal primary neurons with an AMPK inhibitor, compound C, abolished adiponectin-induced neuronal protection. The AMPK activator, 5-aminoimidazole-4-carboxamide-1-beta-D-ribofuranoside, attenuated KA-induced caspase-3 activity. These findings suggest that the AMPK pathway is critically involved in adiponectin-induced neuroprotection and may mediate the antioxidative and anti-apoptotic properties of adiponectin.
Figures
Fig. 1
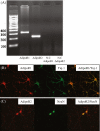
Adiponectin receptors are expressed in hippocampal neurons. a The agarose gel image shows RT-PCR analysis of hippocampal mRNA using primers specific for the receptors AdipoR1 and AdipoR2 (lanes 1 and 2). No PCR products were observed in the two RT negative controls in which reverse transcriptase enzyme were omitted from the cDNA synthesis reaction: lane 3 marked RT used primers for AdipoR1; lane 4 used primers for AdipoR2. b Representative microphotographs illustrating cells positive for both adiponectin receptor 1 (red, left,) and neuronal cell marker Tuj-1 (green, middle). Merged image is shown to the right. c Representative microphotographs illustrating cells positive for both adiponectin receptor 2 (red, left) and neuronal cell marker NeuN (green, middle). Merged image is shown to the right
Fig. 2
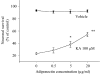
Adiponectin protects hippocampal neurons against excitotoxic death. Hippocampal cultures were pretreated with adiponectin at the indicated concentrations (0.5, 5 and 20 μg/ml) for 48 h and were then exposed to 100 μM KA for 12 h. Neuronal survival was quantified. Values are the mean ± SEM from four different cultures; *p < 0.05, **p < 0.01 compared to the corresponding cultures subjected to KA without adiponectin treatment
Fig. 3
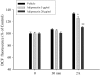
Adiponectin suppresses KA-induced oxidative stress in hippocampal neurons. Hippocampal cultures were pretreated with 5 and 20 μg adiponectin or saline for 48 h and were then exposed to 100 μM KA for 30 min and 2 h. The level of DCF fluorescence was quantified. Values are the means ± SEM from at least six cultures; *p < 0.05, **p < 0.01 compared to the corresponding vehicle-treated control cultures
Fig. 4
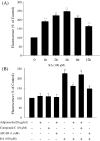
Adiponectin suppresses activation of the excitotoxic apoptosis cascade. a The effects of KA on activation of caspase-3. Hippocampal cultures were subjected to 100 μM KA for the duration indicated. Luminescence analysis showed that caspase-3 activity significantly increased after 1 h KA treatment and reached a peak at 4 h. b The effect of adiponectin on activation of caspase-3 induced by KA. Hippocampal cultures were pretreated with compound C or vehicle, and exposed to 20 μg/ml adiponectin or vehicle (control) or AICAR for 48 h, and then exposed to 100 μM KA or vehicle for 4 h. Levels of luminescence were quantified. Values are the means ± SEM from at least six cultures; *p < 0.05 compared to the cultures treated with KA alone, **p < 0.01 compared to the cultures untreated with KA. Two-way ANOVA was used to find the main effects of KA and five treatments, the latter of which refer to the control, adiponectin, compound C AICAR and combination of adiponectin and compound C groups (data not shown for the last group; KA, F = 281.2, p < 0.001; Five treatments, F = 5.0, p < 0.01; interaction between KA × five treatment, F = 6.0, p < 0.001). Further post hoc test (Student–Newman–Keuls) indicated that the adiponectin and AICAR groups were significantly different from three other groups (p < 0.01)
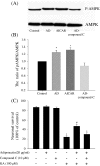
Fig. 5
AMPK phosphorylation modulates the neuroprotective effect of adiponectin. a Hippocampal cultures were pretreated with compound C or vehicle, and exposed to 20 μg/ml adiponectin or vehicle (control) or AICAR for 30 min. Cell lysates were subjected to Western blot analysis using antibodies against p-AMPK and AMPK. b Densitometric analysis of ratios of p-AMPK/AMPK in the hippocampal cultures for four groups; *p < 0.05 compared to the corresponding vehicle-treated controls or the cultures treated with adiponectin and compound C. (C) Hippocampal cultures were pretreated with compound C or vehicle for 2 h and were then treated with adiponectin for 48 h, followed by exposure to 100 μM KA. Neuronal survival was quantified. The values are the means ± SEM from four cultures; *p < 0.01 compared to the hippocampal cultures without any treatment; #p < 0.01 compared to the hippocampal cultures subjected to KA without adiponectin pretreatment or hippocampal cultures subjected to KA with adiponectin and compound C pretreatment. Two-way ANOVA was used to find the main effects of KA and four treatments, the latter of which refer to the control, adiponectin, compound C and combination of adiponectin and compound C groups (data not shown for the last group) (KA, F = 431.9, p < 0.001; four treatments, F = 6.9, p < 0.001; interaction between KA × four treatment, F = 3.1, p < 0.05). Further post hoc test (Student–Newman–Keuls) indicated that the adiponectin group was significantly different from other three groups (p < 0.01)
Similar articles
- Anthocyanins protect against kainic acid-induced excitotoxicity and apoptosis via ROS-activated AMPK pathway in hippocampal neurons.
Ullah I, Park HY, Kim MO. Ullah I, et al. CNS Neurosci Ther. 2014 Apr;20(4):327-38. doi: 10.1111/cns.12218. Epub 2014 Jan 7. CNS Neurosci Ther. 2014. PMID: 24393263 Free PMC article. - Adiponectin attenuates angiotensin II-induced oxidative stress in renal tubular cells through AMPK and cAMP-Epac signal transduction pathways.
Fang F, Liu GC, Kim C, Yassa R, Zhou J, Scholey JW. Fang F, et al. Am J Physiol Renal Physiol. 2013 Jun 1;304(11):F1366-74. doi: 10.1152/ajprenal.00137.2012. Epub 2013 Mar 27. Am J Physiol Renal Physiol. 2013. PMID: 23535586 - Adiponectin protects hippocampal neurons against kainic acid-induced excitotoxicity.
Jeon BT, Shin HJ, Kim JB, Kim YK, Lee DH, Kim KH, Kim HJ, Kang SS, Cho GJ, Choi WS, Roh GS. Jeon BT, et al. Brain Res Rev. 2009 Oct;61(2):81-8. doi: 10.1016/j.brainresrev.2009.05.002. Epub 2009 May 19. Brain Res Rev. 2009. PMID: 19460404 Review. - Ropivacaine Protects against Memory Impairment and Hippocampal Damage in a Rat Neurodegeneration Model.
Chiu KM, Lin TY, Lee MY, Lu CW, Wang MJ, Wang SJ. Chiu KM, et al. Pharmacology. 2018;102(5-6):307-315. doi: 10.1159/000493145. Epub 2018 Sep 26. Pharmacology. 2018. PMID: 30257255 - A pharmacological activator of AMP-activated protein kinase protects hypoxic neurons in a concentration-dependent manner.
Zhang X, Gao R, Li J, Qi Y, Song X, Zhao L, Wang H, Pu Y, Xu K, Li J. Zhang X, et al. Neurochem Res. 2010 Aug;35(8):1281-9. doi: 10.1007/s11064-010-0186-3. Epub 2010 May 20. Neurochem Res. 2010. PMID: 20490918
Cited by
- Targeting Adipokines: A Promising Therapeutic Strategy for Epilepsy.
Shaikh I, Bhatt LK. Shaikh I, et al. Neurochem Res. 2024 Nov;49(11):2973-2987. doi: 10.1007/s11064-024-04219-4. Epub 2024 Jul 26. Neurochem Res. 2024. PMID: 39060767 Review. - Liver-specific adiponectin gene therapy suppresses microglial NLRP3-inflammasome activation for treating Alzheimer's disease.
Ng RC, Jian M, Ma OK, Xiang AW, Bunting M, Kwan JS, Wong CW, Yick LW, Chung SK, Lam KS, Alexander IE, Xu A, Chan KH. Ng RC, et al. J Neuroinflammation. 2024 Mar 27;21(1):77. doi: 10.1186/s12974-024-03066-y. J Neuroinflammation. 2024. PMID: 38539253 Free PMC article. - The Antiviral Potential of AdipoRon, an Adiponectin Receptor Agonist, Reveals the Ability of Zika Virus to Deregulate Adiponectin Receptor Expression.
El Safadi D, Lebeau G, Turpin J, Lefebvre d'Hellencourt C, Diotel N, Viranaicken W, Krejbich-Trotot P. El Safadi D, et al. Viruses. 2023 Dec 22;16(1):24. doi: 10.3390/v16010024. Viruses. 2023. PMID: 38257725 Free PMC article. - Regulatory Basis of Adipokines Leptin and Adiponectin in Epilepsy: from Signaling Pathways to Glucose Metabolism.
Shan Y, Chen Y, Gu H, Wang Y, Sun Y. Shan Y, et al. Neurochem Res. 2023 Jul;48(7):2017-2028. doi: 10.1007/s11064-023-03891-2. Epub 2023 Feb 16. Neurochem Res. 2023. PMID: 36797447 Free PMC article. Review. - Fructose Diet-Associated Molecular Alterations in Hypothalamus of Adolescent Rats: A Proteomic Approach.
D'Ambrosio C, Cigliano L, Mazzoli A, Matuozzo M, Nazzaro M, Scaloni A, Iossa S, Spagnuolo MS. D'Ambrosio C, et al. Nutrients. 2023 Jan 16;15(2):475. doi: 10.3390/nu15020475. Nutrients. 2023. PMID: 36678346 Free PMC article.
References
- Budak E, Fernandez SM, Bellver J, Cervero A, Simon C, Pellicer A (2006) Interactions of the hormones leptin, ghrelin, adiponectin, resistin, and PYY3-36 with the reproductive system. Fertil Steril 85:1563–1581 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials