Diabetes treatment patterns and goal achievement in primary diabetes care (DiaRegis) - study protocol and patient characteristics at baseline - PubMed (original) (raw)
Multicenter Study
Diabetes treatment patterns and goal achievement in primary diabetes care (DiaRegis) - study protocol and patient characteristics at baseline
Peter Bramlage et al. Cardiovasc Diabetol. 2010.
Abstract
Background: Patients with type 2 diabetes are at an increased risk for disease and treatment related complications after the initial approach of oral mono/dual antidiabetic therapy has failed. Data from clinical practice with respect to this patient group are however scarce. Therefore we set up a registry in primary care documenting the course and outcomes of this patient group.
Methods: Diabetes Treatment Patterns and Goal Achievement in Primary Diabetes Care (DiaRegis) is a prospective, observational, German, multicenter registry including patients with type-2 diabetes in which oral mono/dual antidiabetic therapy has failed. Data were recorded at baseline and will be prospectively documented during visits at 6 ± 1, 12 ± 2 and 24 ± 2 months. The primary objective is to estimate the proportion of patients with at least 1 episode of severe hypoglycemia within one year.
Results: 313 primary care offices included 4,048 patients between June 2009 and March 2010 of which 3,810 patients fulfilled the in- and exclusion criteria. 46.7% of patients were female; patients had a median diabetes duration of 5.5 years and most were obese with respect to BMI or waist circumference. HbA1c at baseline was 7.4%, fasting plasma glucose 142 mg/dl and postprandial glucose 185 mg/dl. Co-morbidity in this patient population was substantial with 17.9% having coronary artery disease, 14.4% peripheral neuropathy, 9.9% heart failure and 6.0% peripheral arterial disease. 68.6% of patients received oral monotherapy, 31.4% dual oral combination therapy. The most frequent antidiabetic agent used as monotherapy was metformin (79.0%) followed by sulfonylureas (14.8%).
Conclusions: DiaRegis is a large, prospective registry in primary diabetes care to document the course and outcomes of patients with type-2 diabetes in which the initial approach of oral mono/dual antidiabetic therapy has failed. The two year follow-up will allow for a prospective evaluation of these patients during multiple adjustments of therapy.
Figures
Figure 1
Intensified treatment has been associated with an increase in the rate of hypoglycemia [4-6].
Figure 2
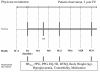
Graphical study design. M, month(s); FU, follow-up; D, Day; FPG, fasting plasma glucose; PPG, post-prandial glucose; EQ-5D, EuroQol-5D; DTSQ, Diabetes Treatment Satisfaction Questionnaire
Figure 3
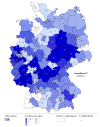
Regional distribution of participating practices throughout Germany (as of 30.04.2010).
Figure 4
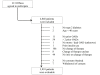
Patient flow chart. OAD, oral antidiabetic drugs
Figure 5
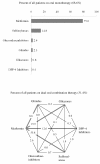
Drug utilization in DiaRegis of patients on oral monotherapy (68.6% of all patients) (panel A) and on dual oral combination therapy (31.4%of all patients) (panel B).
Figure 6
Reason for a change of pharmacotherapy at baseline (panel A) and choice of drugs thereafter (panel B).
Similar articles
- Prognostic implications of DPP-4 inhibitor vs. sulfonylurea use on top of metformin in a real world setting - results of the 1 year follow-up of the prospective DiaRegis registry.
Gitt AK, Bramlage P, Binz C, Krekler M, Deeg E, Tschöpe D. Gitt AK, et al. Int J Clin Pract. 2013 Oct;67(10):1005-14. doi: 10.1111/ijcp.12179. Epub 2013 Aug 28. Int J Clin Pract. 2013. PMID: 23981060 - Hypoglycaemia is more frequent in type 2 diabetic patients with co-morbid vascular disease: an analysis of the DiaRegis registry.
Gitt AK, Bramlage P, Binz C, Krekler M, Plate T, Deeg E, Tschöpe D; DiaRegis Study Group. Gitt AK, et al. Eur J Prev Cardiol. 2012 Aug;19(4):765-72. doi: 10.1177/1741826711411104. Epub 2011 May 31. Eur J Prev Cardiol. 2012. PMID: 21628353 - Repaglinide : a pharmacoeconomic review of its use in type 2 diabetes mellitus.
Plosker GL, Figgitt DP. Plosker GL, et al. Pharmacoeconomics. 2004;22(6):389-411. doi: 10.2165/00019053-200422060-00005. Pharmacoeconomics. 2004. PMID: 15099124 Review. - Oral antidiabetic agents: current role in type 2 diabetes mellitus.
Krentz AJ, Bailey CJ. Krentz AJ, et al. Drugs. 2005;65(3):385-411. doi: 10.2165/00003495-200565030-00005. Drugs. 2005. PMID: 15669880 Review.
Cited by
- A higher TyG index level is more likely to have enhanced incidence of T2DM and HTN comorbidity in elderly Chinese people: a prospective observational study from the reaction study.
Su W, Wang J, Chen K, Yan W, Gao Z, Tang X, Wan Q, Luo Z, Ning G, Mu Y. Su W, et al. Diabetol Metab Syndr. 2024 Jan 30;16(1):29. doi: 10.1186/s13098-024-01258-3. Diabetol Metab Syndr. 2024. PMID: 38287450 Free PMC article. - Achievement of individualized treatment targets in patients with comorbid type-2 diabetes and hypertension: 6 months results of the DIALOGUE registry.
Schmieder RE, Gitt AK, Koch C, Bramlage P, Ouarrak T, Tschöpe D; DIALOGUE study group. Schmieder RE, et al. BMC Endocr Disord. 2015 May 2;15:23. doi: 10.1186/s12902-015-0020-7. BMC Endocr Disord. 2015. PMID: 25934177 Free PMC article. - A real world comparison of sulfonylurea and insulin vs. incretin-based treatments in patients not controlled on prior metformin monotherapy.
Gitt AK, Bramlage P, Schneider S, Tschöpe D. Gitt AK, et al. Cardiovasc Diabetol. 2015 Feb 3;14:13. doi: 10.1186/s12933-015-0172-9. Cardiovasc Diabetol. 2015. PMID: 25645672 Free PMC article. - Antidiabetic treatment patterns in a medicare advantage population in the United States.
Slabaugh SL, Xu Y, Stacy JN, Baltz JC, Meah YA, Lian J, Moretz DC, Bouchard JR. Slabaugh SL, et al. Drugs Aging. 2015 Feb;32(2):169-78. doi: 10.1007/s40266-014-0235-8. Drugs Aging. 2015. PMID: 25573537 - Clinical course and outcomes of type-2 diabetic patients after treatment intensification for insufficient glycaemic control - results of the 2 year prospective DiaRegis follow-up.
Bramlage P, Gitt AK, Schneider S, Deeg E, Tschöpe D; DiaRegis Study Group. Bramlage P, et al. BMC Cardiovasc Disord. 2014 Nov 19;14:162. doi: 10.1186/1471-2261-14-162. BMC Cardiovasc Disord. 2014. PMID: 25410473 Free PMC article.
References
- Matthaei S, Bierwirth R, Fritsche A, Gallwitz B, Haring HU, Joost HG, Kellerer M, Kloos C, Kunt T, Nauck M, Schernthaner G, Siegel E, Thienel F. Medical antihyperglycaemic treatment of type 2 diabetes mellitus: update of the evidence-based guideline of the German Diabetes Association. Exp Clin Endocrinol Diabetes. 2009;9(9):522–557. doi: 10.1055/s-0029-1239559. - DOI - PubMed
- Gerstein HC, Miller ME, Byington RP, Goff DC Jr, Bigger JT, Buse JB, Cushman WC, Genuth S, Ismail-Beigi F, Grimm RH Jr, Probstfield JL, Simons-Morton DG, Friedewald WT. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;9(24):2545–2559. doi: 10.1056/NEJMoa0802743. - DOI - PMC - PubMed
- Patel A, MacMahon S, Chalmers J, Neal B, Billot L, Woodward M, Marre M, Cooper M, Glasziou P, Grobbee D, Hamet P, Harrap S, Heller S, Liu L, Mancia G, Mogensen CE, Pan C, Poulter N, Rodgers A, Williams B, Bompoint S, de Galan BE, Joshi R, Travert F. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;9(24):2560–2572. doi: 10.1056/NEJMoa0802987. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical