The sphingosine 1-phosphate receptor, S1PR₁, plays a prominent but not exclusive role in enhancing the excitability of sensory neurons - PubMed (original) (raw)
The sphingosine 1-phosphate receptor, S1PR₁, plays a prominent but not exclusive role in enhancing the excitability of sensory neurons
Xian Xuan Chi et al. J Neurophysiol. 2010 Nov.
Abstract
Sphingosine 1-phosphate (S1P) through its interaction with a family of G protein-coupled receptors (S1PR) is proving to have a significant impact on the activation of a variety of cell types, most notably those cells mediating the inflammatory response. Previously, we showed that S1P enhanced the excitability of small diameter sensory neurons, and mRNA for S1PR(1-4) was expressed in sensory neurons. These initial findings did not determine which S1PR subtype(s) mediated the increased excitability. Here, we report that exposure to the selective S1PR(1) agonist, SEW2871, produced a significant increase in excitability of some, but not all, sensory neurons. To further examine the role of S1PR(1), neurons were treated with siRNA targeted to S1PR(1). siRNA reduced S1PR(1) protein expression by 75% and blocked the sensitization produced by SEW2871, although some neurons remained responsive to subsequent exposure to S1P. Treatment with scramble siRNA did not alter S1PR(1) expression. Recordings from siRNA- and scramble-treated neurons suggested three distinct populations based on their sensitivities to SEW2871 and S1P. Approximately 50% of the neurons exhibited a significant increase in excitability after exposure to SEW2871 and subsequent S1P produced no additional increase; ∼25% were not affected by SEW2871 but S1P significantly increased excitability; and ∼25% of the neurons were not sensitized by either SEW2871 or S1P. RT-PCR measurements obtained from single neurons showed that 50% of the small diameter neurons expressed the mRNA for S1PR(1). These results indicate that S1PR(1) plays a prominent, although not exclusive, role in mediating the enhancement of excitability produced by S1P.
Figures
Fig. 1.
Sphingosine 1-phosphate (S1P) enhances the excitability of small diameter sensory neurons. A: representative voltage traces recorded under control conditions (left) and after a 5-min exposure to 1 μM S1P (right). Under control conditions, this small diameter sensory neuron had a resting membrane potential of −49 mV, and 3 action action potentials (APs) were evoked by the ramp of depolarizing current (450 pA, bottom trace). After S1P, the resting potential was depolarized to −42 mV and now the ramp evoked 12 APs. The dashed line indicates the 0 mV level. B: representative voltage recordings obtained from the same neuron as in A for steps of depolarizing current. Left: response to a 50- (top) and 300-pA (bottom) step of current. Right: response to the same current steps after a 5-min exposure to 1 μM S1P. C: the time-dependent increase in excitability produced by S1P in 5 sensory neurons for APs elicited by the ramps of depolarizing currents. The asterisks indicate a significant difference compared with the control condition with P < 0.05, RM ANOVA.
Fig. 2.
The S1P receptor (S1PR1) selective agonist, SEW2871, increases the number of evoked APs in some, but not all, sensory neurons. In 5 of 9 neurons, 100 nM SEW2871 produced a significant increase in the number of APs evoked by the ramp of current, whereas in 4 of the 9, SEW2871 had no effect. We define sensitivity to SEW2871 as a >2-fold increase in the number of evoked APs after exposure. The asterisks indicate a significant difference compared with the control condition with P < 0.05, RM ANOVA.
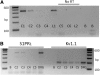
Fig. 3.
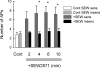
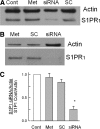
siRNA targeted to S1PR1 greatly reduces the protein expression of this receptor. A: a representative Western blot where the expression of actin and S1PR1 were measured for 4 different conditions. The untreated control is indicated by the lane labeled Cont, exposure to only the transfecting detergent metafectene is labeled Met, treatment with 200 nM siRNA targeted to S1PR1 is labeled siRNA, and treatment with 200 nM scramble siRNA is labeled SC. The antibody for actin was used at a dilution of 1:1,000; the antibody to S1PR1 was used at a dilution of 1:200. B: another representative Western blot where the amount of protein added to the lane labeled siRNA was doubled for detection purposes. C: levels of expression of S1PR1 for the 4 treatment conditions obtained from 5 different tissue harvests: untreated control condition (Cont), exposure to only metafectene (Met), treatment with 200 nM scramble siRNA (SC), and treatment with 200 nM siRNA targeted to S1PR1 (siRNA). The densities for the protein bands for S1PR1 under these different treatment conditions have been normalized to their respective levels for actin densities, and all values have been normalized to their respective untreated controls. The asterisk indicates a significant difference compared with the control condition with P < 0.05, ANOVA.
Fig. 4.
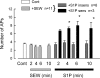
siRNA targeted to S1PR1 blocks the sensitization to SEW2871, although S1P can still sensitize some neurons. After neurons were treated with 200 nM siRNA targeted to S1PR1 (fluorescently labeled) using our standard protocol, they were used for recordings. The 11 small diameter sensory neurons that were exposed to 100 nM SEW2871 failed to exhibit an increase in AP firing by the ramp over a 10-min period. Of the 11 neurons, 9 were exposed to 1 μM S1P for another 10 min. Six of the 9 neurons remained unaffected after exposure to S1P, whereas 3 of the 9 exhibited increased firing after S1P. The asterisks indicate a significant difference compared with the 10-min SEW2871 condition with P < 0.05, ANOVA.
Fig. 5.
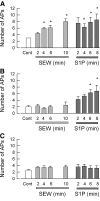
In scramble siRNA-treated sensory neurons, SEW2871 and S1P have varying capacities to augment neuronal excitability. Sensory neurons were treated with 200 nM scramble siRNA that was fluorescently labeled using our standard protocol. Recordings were obtained from a total of 16 small diameter neurons during exposure to 100 nM SEW2871 for 10 min and then to 1 μM S1P for an additional 10 min. A: results obtained from 7 of the 16 neurons wherein SEW2871 significantly increased AP firing and that S1P produced no additional effect. B: results obtained from 4 of the 16 neurons wherein SEW2871 was ineffective, but S1P did produce a significant increase in AP firing. C: 5 of the 16 neurons were not affected by either SEW2871 or S1P. The asterisks indicate a significant difference compared with the control condition with P < 0.05, ANOVA.
Fig. 6.
Single-cell RT-PCR analysis shows that some, but not all, small diameter sensory neurons express the mRNA for S1PR1. A: representative gel for the detection of S1PR1 in 5 individual sensory neurons. The lanes labeled C1–C6 (cell1–cell6) are the mRNAs from individual small diameter neurons, whereas those labeled L1–L2 (large cell1 and cell2) are mRNAs obtained from individual large diameter neurons. Lanes C1–L1 represent the detection of S1PR1 amplicons with a product size of 448 bp. Lanes C5–L2 underwent PCR in the absence of RT (No RT). Lanes labeled B (blank) represent reactions performed in the absence of any template. B: in a different tissue harvest than shown in A, the detection of S1PR1 and the potassium channel, Kv1.1 from the same 4 individual sensory neurons. For small diameter sensory neurons C2–C5, Kv1.1 amplicons were detected, whereas S1PR1 was detected in only 2 of the 4 neurons. The lanes labeled DRG were amplicons obtained from mRNA isolated from 10 ganglia.
Similar articles
- Sphingosine 1-phosphate enhances the excitability of rat sensory neurons through activation of sphingosine 1-phosphate receptors 1 and/or 3.
Li C, Li JN, Kays J, Guerrero M, Nicol GD. Li C, et al. J Neuroinflammation. 2015 Apr 12;12:70. doi: 10.1186/s12974-015-0286-8. J Neuroinflammation. 2015. PMID: 25880547 Free PMC article. - Sphingosine 1-phosphate receptor 2 antagonist JTE-013 increases the excitability of sensory neurons independently of the receptor.
Li C, Chi XX, Xie W, Strong JA, Zhang JM, Nicol GD. Li C, et al. J Neurophysiol. 2012 Sep;108(5):1473-83. doi: 10.1152/jn.00825.2011. Epub 2012 Jun 6. J Neurophysiol. 2012. PMID: 22673325 Free PMC article. - Sphingosine-1-phosphate promotes the proliferation and attenuates apoptosis of Endothelial progenitor cells via S1PR1/S1PR3/PI3K/Akt pathway.
Wang H, Huang H, Ding SF. Wang H, et al. Cell Biol Int. 2018 Nov;42(11):1492-1502. doi: 10.1002/cbin.10991. Epub 2018 Jul 8. Cell Biol Int. 2018. PMID: 29790626 - New players on the center stage: sphingosine 1-phosphate and its receptors as drug targets.
Huwiler A, Pfeilschifter J. Huwiler A, et al. Biochem Pharmacol. 2008 May 15;75(10):1893-900. doi: 10.1016/j.bcp.2007.12.018. Epub 2008 Jan 5. Biochem Pharmacol. 2008. PMID: 18321471 Review. - Insight into modulators of sphingosine-1-phosphate receptor and implications for cardiovascular therapeutics.
Xie YX, Yao H, Peng JF, Ni D, Liu WT, Li CQ, Yi GH. Xie YX, et al. J Drug Target. 2024 Dec;32(3):300-310. doi: 10.1080/1061186X.2024.2309577. Epub 2024 Feb 6. J Drug Target. 2024. PMID: 38269855 Review.
Cited by
- Density of Sphingosine-1-Phosphate Receptors Is Altered in Cortical Nerve-Terminals of Insulin-Resistant Goto-Kakizaki Rats and Diet-Induced Obese Mice.
Skoug C, Erdogan H, Vanherle L, Vieira JPP, Matthes F, Eliasson L, Meissner A, Duarte JMN. Skoug C, et al. Neurochem Res. 2024 Feb;49(2):338-347. doi: 10.1007/s11064-023-04033-4. Epub 2023 Oct 4. Neurochem Res. 2024. PMID: 37794263 Free PMC article. - miRNA-130a-3p targets sphingosine-1-phosphate receptor 1 to activate the microglial and astrocytes and to promote neural injury under the high glucose condition.
Yang G, Shi J. Yang G, et al. Open Med (Wars). 2022 Dec 20;17(1):2117-2129. doi: 10.1515/med-2022-0565. eCollection 2022. Open Med (Wars). 2022. PMID: 36582210 Free PMC article. - Sphingosine-1-phosphate receptor 1 activation in the central nervous system drives cisplatin-induced cognitive impairment.
Squillace S, Niehoff ML, Doyle TM, Green M, Esposito E, Cuzzocrea S, Arnatt CK, Spiegel S, Farr SA, Salvemini D. Squillace S, et al. J Clin Invest. 2022 Sep 1;132(17):e157738. doi: 10.1172/JCI157738. J Clin Invest. 2022. PMID: 36047496 Free PMC article. - Targeting the Sphingosine-1-Phosphate Axis for Developing Non-narcotic Pain Therapeutics.
Squillace S, Spiegel S, Salvemini D. Squillace S, et al. Trends Pharmacol Sci. 2020 Nov;41(11):851-867. doi: 10.1016/j.tips.2020.09.006. Epub 2020 Oct 1. Trends Pharmacol Sci. 2020. PMID: 33010954 Free PMC article. Review. - Metabolic tuning of inhibition regulates hippocampal neurogenesis in the adult brain.
Wang X, Liu H, Morstein J, Novak AJE, Trauner D, Xiong Q, Yu Y, Ge S. Wang X, et al. Proc Natl Acad Sci U S A. 2020 Oct 13;117(41):25818-25829. doi: 10.1073/pnas.2006138117. Epub 2020 Sep 24. Proc Natl Acad Sci U S A. 2020. PMID: 32973092 Free PMC article.
References
- Allende ML, Dreier JL, Mandala S, Proia RL. Expression of the sphingosine 1-phosphate receptor, S1P1, on T-cells controls thymic emigration. J Biol Chem 279: 15396–15401, 2004 - PubMed
- Anelli V, Bassi R, Tettamanti G, Viani P, Riboni L. Extracellular release of newly synthesized sphingosine-1-phosphate by cerebellar granule cells and astrocytes. J Neurochem 92: 1204–1215, 2005 - PubMed
- Anliker B, Chun J. Cell surface receptors in lysophospholipid signaling. Semin Cell Dev Biol 15: 457–465, 2004 - PubMed
- Brinkmann V, Davis MD, Heise CE, Albert R, Cottens S, Hof R, Bruns C, Prieschl E, Baumruker T, Hiestand P, Foster CA, Zollinger M, Lynch KR. The immune modulator FTY720 targets sphingosine 1-phosphate receptors. J Biol Chem 277: 21453–21457, 2002 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 NS046084/NS/NINDS NIH HHS/United States
- R01 NS-046084/NS/NINDS NIH HHS/United States
- R01 NS060853/NS/NINDS NIH HHS/United States
- C06 RR015481-01/RR/NCRR NIH HHS/United States
- R01 NS-060853/NS/NINDS NIH HHS/United States
- C06 RR015481/RR/NCRR NIH HHS/United States
LinkOut - more resources
Full Text Sources