PEG-functionalized magnetic nanoparticles for drug delivery and magnetic resonance imaging applications - PubMed (original) (raw)
PEG-functionalized magnetic nanoparticles for drug delivery and magnetic resonance imaging applications
Murali Mohan Yallapu et al. Pharm Res. 2010 Nov.
Abstract
Purpose: Polyethylene glycol (PEG) functionalized magnetic nanoparticles (MNPs) were tested as a drug carrier system, as a magnetic resonance imaging (MRI) agent, and for their ability to conjugate to an antibody.
Methods: An iron oxide core coated with oleic acid (OA) and then with OA-PEG forms a water-dispersible MNP formulation. Hydrophobic doxorubicin partitions into the OA layer for sustained drug delivery. The T(1) and T(2) MRI contrast properties were determined in vitro and the circulation of the MNPs was measured in mouse carotid arteries. An N-hydroxysuccinimide group (NHS) on the OA-PEG-80 was used to conjugate the amine functional group on antibodies for active targeting in the human MCF-7 breast cancer cell line.
Results: The optimized formulation had a mean hydrodynamic diameter of 184 nm with an ~8 nm iron-oxide core. The MNPs enhance the T(2) MRI contrast and have a long circulation time in vivo with 30% relative concentration 50 min post-injection. Doxorubicin-loaded MNPs showed sustained drug release and dose-dependent antiproliferative effects in vitro; the drug effect was enhanced with transferrin antibody-conjugated MNPs.
Conclusion: PEG-functionalized MNPs could be developed as a targeted drug delivery system and MRI contrast agent.
Figures
Figure 1
Magnetic nanoparticle synthesis (A) and layering of various OA-PEG polymers on the surface of the magnetic nanoparticles (B).
Figure 2
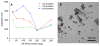
Magnetic nanoparticle size changes with OA-PEG polymer coating. (A) Mean particle size in water after modification with 25–300 mg of OE-20, OE-40, or OE-80 polymers. (B) Representative transmission electron micrograph of OE-40 polymer coated MNPs. Diameter of iron-oxide core ~8 nm.
Figure 3
Polymer modifications to OA-MNP core confirmed by (A) FTIR and (B) TGA.
Figure 4
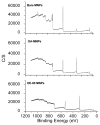
X-ray photoelectron spectra with plain, OA, and OA and PEG-OE-80 coatings.
Figure 5
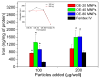
Macrophage uptake of particles with respect to iron level in protein. (Inset) Particle size in RPMI media (data is represented as mean ± SEM, n=3, *p<0.05 compared to Feridex IV).
Figure 6
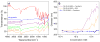
Magnetic resonance imaging. (A) Contrast at varying iron concentrations for OA-PEG-MNPs and Feridex IV in agar gels. T2 relaxivity (B) and T1 relaxivity (C) for OE-80 MNPs. (Data as values obtained from curve fitting and standard errors are uncertainties in fitting.)
Figure 7
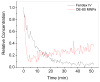
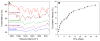
Calculated relative iron concentration vs. time profiles of magnetic nanoparticles in a mouse carotid artery. Shown is the change in one carotid artery but both carotid arteries, and MNP injections in additional mice, showed an almost identical pattern.
Figure 8
Characterization and drug release of DOX-MNPs. (A) Polymer formulations modified with DOX and (B) release profile of DOX from DOX-OE-80 MNPs (data is represented as mean ± SEM, n=3).
Figure 9
Transferrin antibody conjugation to OE-80 MNPs. (A) Antibody conjugation to plain and DOX loaded OE-80 MNPs. (B) Transferrin and BSA binding to OE-80-Ab MNPs indicates proper orientation of transferrin antibody antigen binding sites (data is represented as mean ± SEM, n=3).
Similar articles
- Polyethylene glycol modified, cross-linked starch-coated iron oxide nanoparticles for enhanced magnetic tumor targeting.
Cole AJ, David AE, Wang J, Galbán CJ, Hill HL, Yang VC. Cole AJ, et al. Biomaterials. 2011 Mar;32(8):2183-93. doi: 10.1016/j.biomaterials.2010.11.040. Epub 2010 Dec 21. Biomaterials. 2011. PMID: 21176955 Free PMC article. - Controlled release of doxorubicin from polyethylene glycol functionalized melanin nanoparticles for breast cancer therapy: Part I. Production and drug release performance of the melanin nanoparticles.
Ozlu B, Kabay G, Bocek I, Yilmaz M, Piskin AK, Shim BS, Mutlu M. Ozlu B, et al. Int J Pharm. 2019 Oct 30;570:118613. doi: 10.1016/j.ijpharm.2019.118613. Epub 2019 Aug 12. Int J Pharm. 2019. PMID: 31415880 - Magnetic nanoparticles with dual functional properties: drug delivery and magnetic resonance imaging.
Jain TK, Richey J, Strand M, Leslie-Pelecky DL, Flask CA, Labhasetwar V. Jain TK, et al. Biomaterials. 2008 Oct;29(29):4012-21. doi: 10.1016/j.biomaterials.2008.07.004. Epub 2008 Jul 22. Biomaterials. 2008. PMID: 18649936 Free PMC article. - Lipid-polymer hybrid nanoparticles as a new generation therapeutic delivery platform: a review.
Hadinoto K, Sundaresan A, Cheow WS. Hadinoto K, et al. Eur J Pharm Biopharm. 2013 Nov;85(3 Pt A):427-43. doi: 10.1016/j.ejpb.2013.07.002. Epub 2013 Jul 17. Eur J Pharm Biopharm. 2013. PMID: 23872180 Review. - Design and fabrication of magnetic nanoparticles for targeted drug delivery and imaging.
Veiseh O, Gunn JW, Zhang M. Veiseh O, et al. Adv Drug Deliv Rev. 2010 Mar 8;62(3):284-304. doi: 10.1016/j.addr.2009.11.002. Epub 2009 Nov 10. Adv Drug Deliv Rev. 2010. PMID: 19909778 Free PMC article. Review.
Cited by
- Combining Inulin Multifunctional Polycation and Magnetic Nanoparticles: Redox-Responsive siRNA-Loaded Systems for Magnetofection.
Sardo C, Craparo EF, Porsio B, Giammona G, Cavallaro G. Sardo C, et al. Polymers (Basel). 2019 May 15;11(5):889. doi: 10.3390/polym11050889. Polymers (Basel). 2019. PMID: 31096623 Free PMC article. - Carbon Coated Iron-Cobalt Nanoparticles for Magnetic Particle Imaging.
Kumar R, Huda MN, Habib A, Nafiujjaman M, Woo HJ, Kim T, Nurunnabi M. Kumar R, et al. ACS Appl Bio Mater. 2023 Aug 21;6(8):3257-3265. doi: 10.1021/acsabm.3c00354. Epub 2023 Aug 9. ACS Appl Bio Mater. 2023. PMID: 37554053 Free PMC article. - Composite Hydrogels with Included Solid-State Nanoparticles Bearing Anticancer Chemotherapeutics.
Zhivkov AM, Popov TT, Hristova SH. Zhivkov AM, et al. Gels. 2023 May 17;9(5):421. doi: 10.3390/gels9050421. Gels. 2023. PMID: 37233012 Free PMC article. Review. - Coating Dependent In Vitro Biocompatibility of New Fe-Si Nanoparticles.
Balas M, Dumitrache F, Badea MA, Fleaca C, Badoi A, Tanasa E, Dinischiotu A. Balas M, et al. Nanomaterials (Basel). 2018 Jul 5;8(7):495. doi: 10.3390/nano8070495. Nanomaterials (Basel). 2018. PMID: 29976868 Free PMC article. - Manganese-loaded lipid-micellar theranostics for simultaneous drug and gene delivery to lungs.
Howell M, Mallela J, Wang C, Ravi S, Dixit S, Garapati U, Mohapatra S. Howell M, et al. J Control Release. 2013 Apr 28;167(2):210-8. doi: 10.1016/j.jconrel.2013.01.029. Epub 2013 Feb 6. J Control Release. 2013. PMID: 23395689 Free PMC article.
References
- Bulte JW, Kraitchman DL. Monitoring cell therapy using iron oxide MR contrast agents. Curr Pharm Biotechnol. 2004;5:567–584. - PubMed
- Josephson L. Magnetic Nanoparticles for MR Imaging. Springer; US: 2007.
- Bulte JW, Kraitchman DL. Iron oxide MR contrast agents for molecular and cellular imaging. NMR Biomed. 2004;17:484–499. - PubMed
- Alexiou C, Arnold W, Klein RJ, Parak FG, Hulin P, Bergemann C, Erhardt W, Wagenpfeil S, Lubbe AS. Locoregional cancer treatment with magnetic drug targeting. Cancer Res. 2000;60:6641–6648. - PubMed
- Beaven GH, Chen SH, d’Albis A, Gratzer WB. A spectroscopic study of the haemin--human-serum-albumin system. Eur J Biochem. 1974;41:539–546. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical